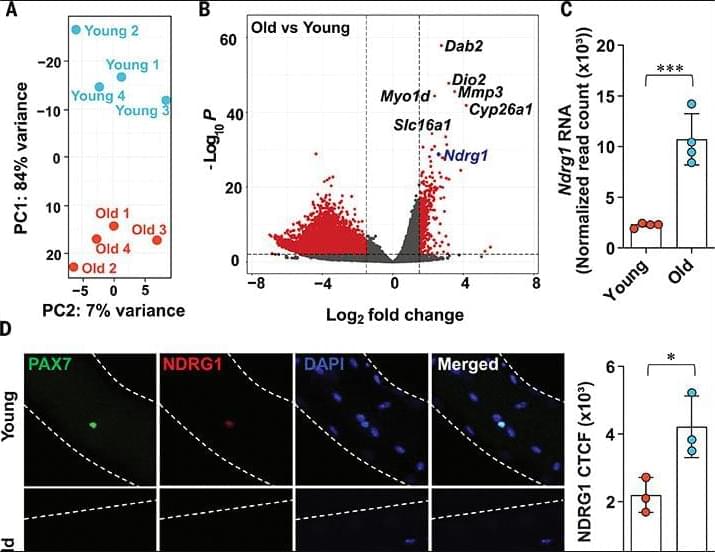
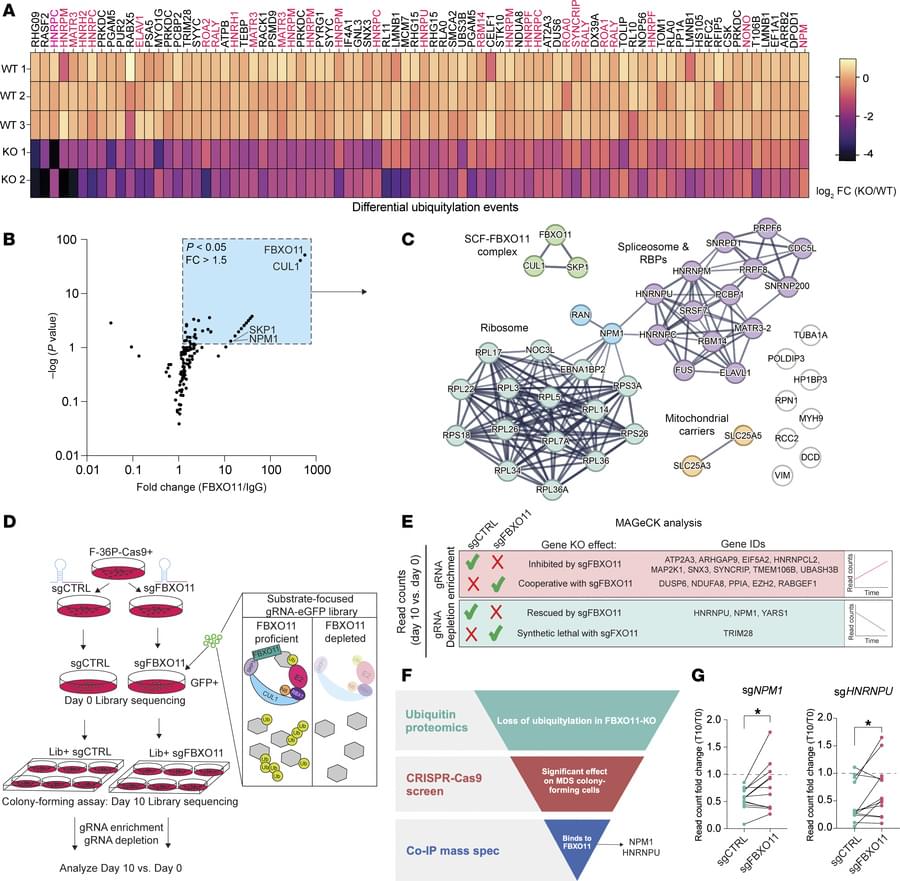
Aging is characterized by a decline in the ability of tissue repair and regeneration after injury. In skeletal muscle, this decline is largely driven by impaired function of muscle stem cells (MuSCs) to efficiently contribute to muscle regeneration. We uncovered a cause of this aging-associated dysfunction: a cellular survivorship bias that prioritizes stem cell persistence at the expense of functionality. With age, MuSCs increased expression of a tumor suppressor, N-myc down-regulated gene 1 (NDRG1), which, by suppressing the mammalian target of rapamycin (mTOR) pathway, increased their long-term survival potential but at the cost of their ability to promptly activate and contribute to muscle regeneration. This delayed muscle regeneration with age may result from a trade-off that favors long-term stem cell survival over immediate regenerative capacity.