Donahue et al. show that ageing is associated with changes in ER morphology. ER-phagy drives age-associated ER remodelling through tissue-specific factors.



DNA methylation plays a critical role in gene expression regulation and has emerged as a robust biomarker of biological age. This modification will become heavier or site drift along with aging. Recently, it is termed epigenetic clocks—such as Horvath, Hannum, PhenoAge, and GrimAge—leverage specific methylation patterns to accurately predict age-related decline, disease risk, and mortality. These tools are now widely applied across diverse tissues, populations, and disease contexts. Beyond age-related loss of methylation control, accelerated DNA methylation age has been linked to environmental exposures, lifestyle factors, and chronic diseases, further reinforcing its value as a dynamic and clinically relevant marker of biological aging. DNA methylation is reshaping our understanding of aging and disease risk, with promising implications for preventive medicine and interventions aimed at promoting healthy longevity. However, it must be admitted that some challenges remain, including limited generalizability across populations, an unclear mechanism, and inconsistent longitudinal performance. In this review, we examine the biological foundations of DNA methylation, major advances in epigenetic clock development, and their expanding applications in aging research, disease prediction and health monitoring.
Aging is a complex, multifactorial process that affects nearly all biological systems. While chronological age simply measures the passage of time from birth, biological age reflects the functional state and health of an individual’s tissues and organs (Kiselev et al., 2025). This distinction is critical, as individuals of the same chronological age often exhibit markedly different biological conditions, disease risks, and mortality trajectories (Dugue et al., 2018). Therefore, biological age potentially serves as a more meaningful measure of aging-related decline and is increasingly used to assess overall health status, predict disease onset, and evaluate the effectiveness of interventions aimed at promoting healthy longevity (Dugue et al., 2018; Petkovich et al., 2017).
Among various biomarkers proposed to estimate biological age, epigenetic modifications—particularly DNA methylation—have emerged as one of the most reliable and informative (Dugue et al., 2018). In epigenetics, DNA methylation involves the addition of a methyl group to the 5′ position of cytosine residues, typically at CpG dinucleotides, which can regulate gene expression without altering the underlying DNA sequence. Moreover, DNA methylation can be accurately measured by sequencing at methylated sites with bisulfate treatment (Zhang et al., 2012). Age-related changes in DNA methylation pattern are not random; they occur at specific genomic locations. These methylated sites are picked and constitute come patterns, by which scientists can construct “epigenetic clocks” to precisely estimate a person’s biological age based on their DNA modification. As people grow older, their methylation profiles shift in predictable ways (Kiselev et al., 2025; Horvath, 2013; Horvath and Raj, 2018).

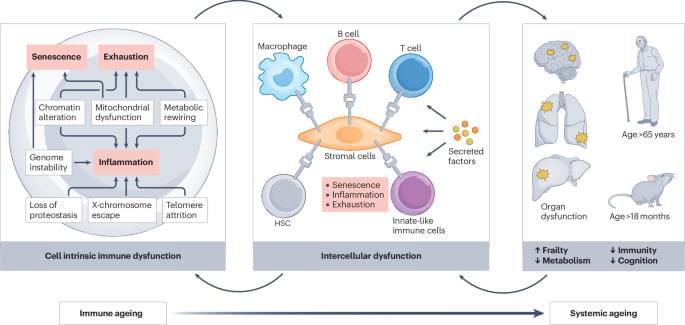
Ageing of the immune system is now realized to drive systemic ageing, and there is interest in targeting immune ageing in order to promote healthy ageing. Here, the authors detail how ageing affects different immune cell populations and discuss strategies to rejuvenate the immune system in order to extend healthspan.

Getting older might seem like a slow, gradual process – but research suggests that this is not always the case.
In fact, if you wake up one morning, look in the mirror, and wonder if your aging somehow accelerated, you might not be imagining things.
According to a 2024 study into the molecular changes associated with aging, humans experience two abrupt lurches forward, one at the average age of 44 and the other at around age 60.
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
Blood testing with LifeExtension.com: https://www.anrdoezrs.net/click-101614996-15750394
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1

Factors that promote inflammation in C9ORF72 mutation carriers with amyotrophic lateral sclerosis (ALS) or frontotemporal dementia (FTD) have remained elusive. McCourt et al. identified pro-inflammatory forms of glycogen in gut contents of people with ALS/FTD and demonstrate that targeting glycogen in a C9orf72 mouse model extends lifespan and reduces neuroinflammation.

Humans develop sharp vision during early fetal development thanks to an interplay between a vitamin A derivative and thyroid hormones in the retina, Johns Hopkins University scientists have found. The findings could upend decades of conventional understanding of how the eye grows light-sensing cells and could inform new research into treatments for macular degeneration, glaucoma, and other age-related vision disorders. Details of the study, which used lab-grown retinal tissue, are published today in Proceedings of the National Academy of Sciences.
“This is a key step toward understanding the inner workings of the center of the retina, a critical part of the eye and the first to fail in people with macular degeneration,” said Robert J. Johnston Jr., an associate professor of biology at Johns Hopkins who led the research. “By better understanding this region and developing organoids that mimic its function, we hope to one day grow and transplant these tissues to restore vision.”
What if worn-out parts of your body didn’t need to be replaced just regenerated?
Stanford researchers recently published work showing cartilage lost to aging or arthritis can be regrown, using either an oral drug or a local injection.
If this translates to humans, it could make knee and hip replacements unnecessary.
Millions of people undergo major joint replacement surgery every year.
Regenerating cartilage instead of replacing joints would mean less pain, lower cost, and far better outcomes.
That future may be closer than we think.