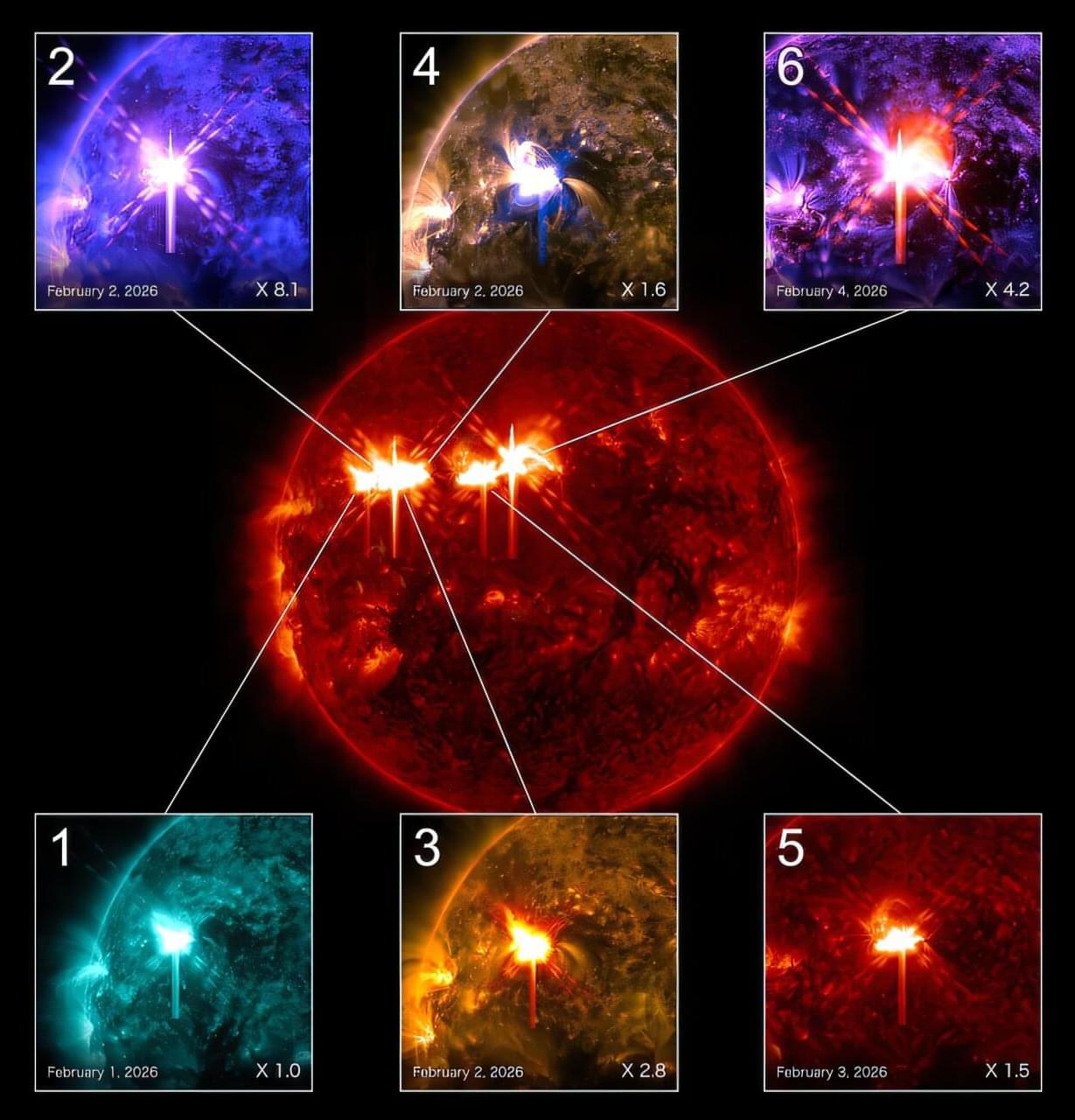
Co-author Dr. Willie Soon, from the Center for Environmental Research and Earth Sciences (CERES), added, “Nature gave us the perfect test. These far-side discoveries essentially validated our method in real time, proving that the underlying patterns we identified are reliable and work everywhere on the sun’s surface.”
Solar superflares are the most powerful eruptions the sun can produce. A direct hit from one of these storms could cause widespread power outages, damage satellites, disrupt GPS navigation, interfere with radio communications, and create radiation hazards for astronauts and airline passengers at high altitudes.