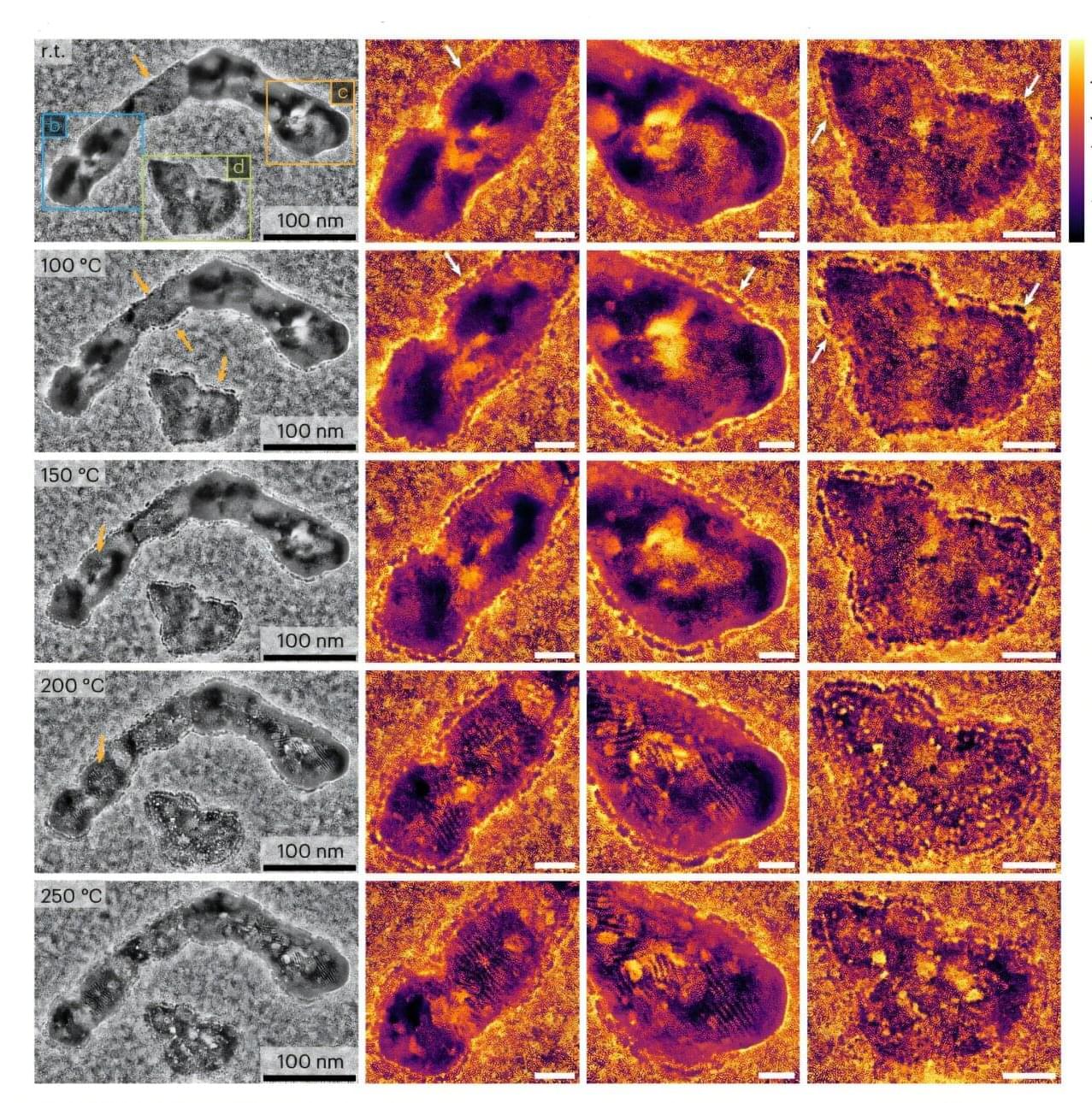
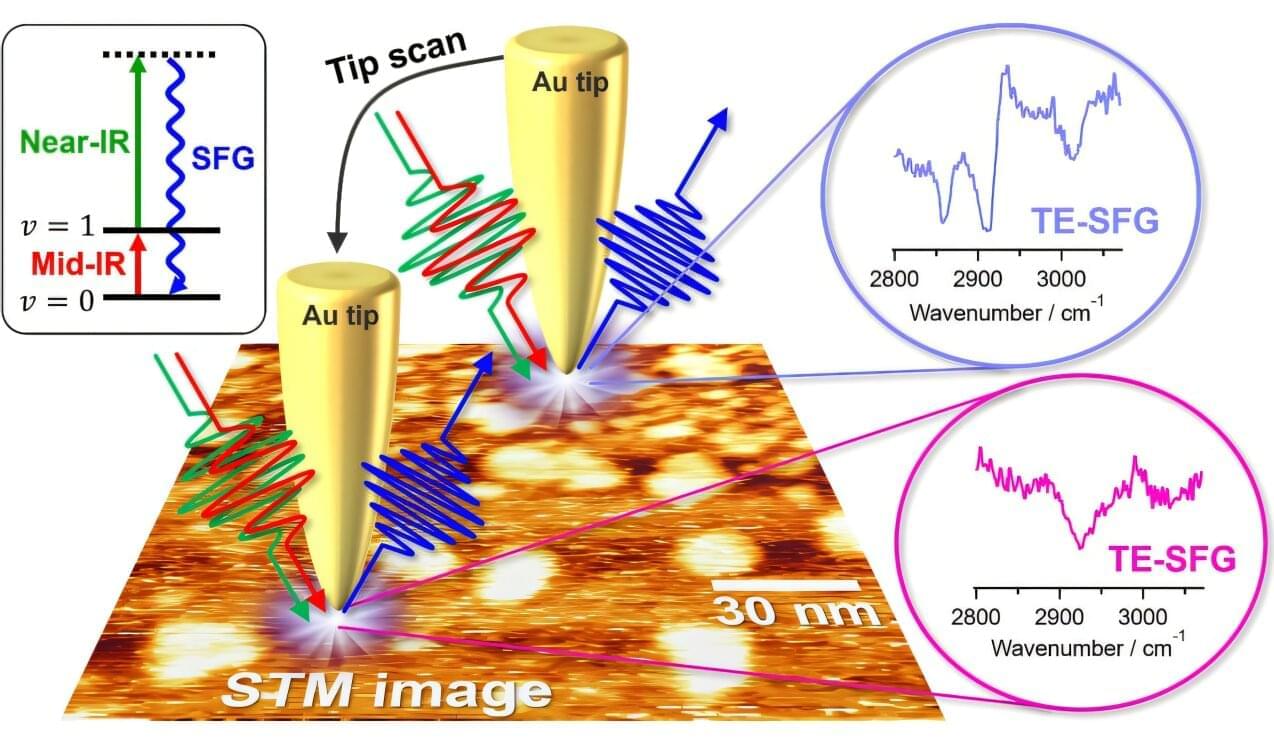
Physicists have uncovered a link between magnetism and a mysterious phase of matter called the pseudogap, which appears in certain quantum materials just above the temperature at which they become superconducting. The findings could help researchers design new materials with sought-after properties such as high-temperature superconductivity, in which electric current flows without resistance.
Using a quantum simulator chilled to just above absolute zero, the researchers discovered a universal pattern in how electrons—which can have spin up or down—influence their neighbors’ spins as the system is cooled.
The findings represent a significant step toward understanding unconventional superconductivity, and were the result of a collaboration between experimentalists at the Max Planck Institute of Quantum Optics in Germany and theoretical physicists, including Antoine Georges, director of the Center for Computational Quantum Physics (CCQ) at the Simons Foundation’s Flatiron Institute in New York City.