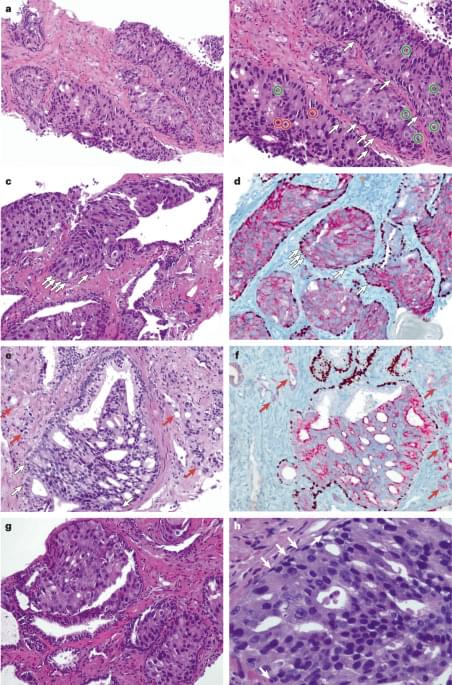
Atypical intraductal proliferation is a prostate biopsy finding with morphological features intermediate between high-grade prostatic intraepithelial neoplasia and intraductal carcinoma. This Review discusses histopathological features, molecular characteristics and clinical outcomes associated with AIP, and highlights the clinical implications of AIP, primarily due to the frequent association of AIP with clinically significant prostate cancer.