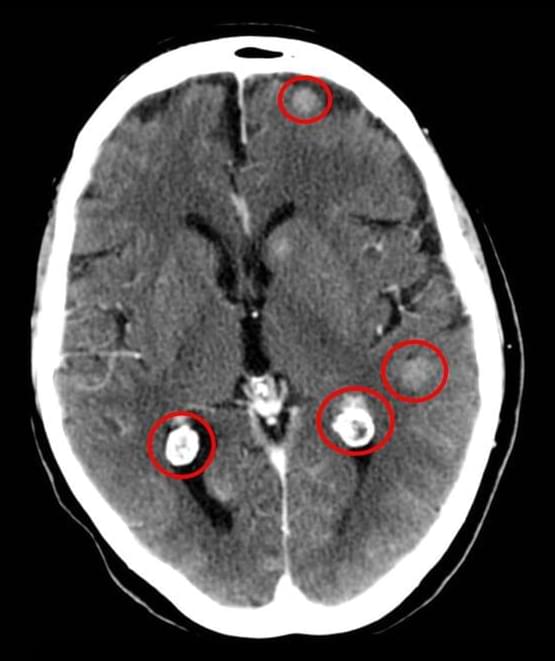
The drug lorlatinib (Lorbrena) is superior to crizotinib (Xalkori) as an initial treatment for people with advanced non-small cell lung cancer (NSCLC) that has changes in the ALK gene, according to new results from a global clinical trial.
The findings are the latest from the CROWN study. Participants were randomly assigned to receive either lorlatinib or crizotinib as a treatment for advanced lung tumors with ALK gene mutations, a disease called ALK-positive lung cancer.
Several years ago, study investigators reported that participants who received lorlatinib went longer without the disease worsening, known as progression-free survival, than those who received crizotinib.