Researchers at NYU Abu Dhabi have developed a new light-based nanotechnology that could improve how certain cancers are detected and treated, offering a more precise and potentially less harmful alternative to chemotherapy, radiation, and surgery. The study advances photothermal therapy, a treatment approach that uses light to generate heat inside tumors and destroy cancer cells.
The research is published in the journal Cell Reports Physical Science.
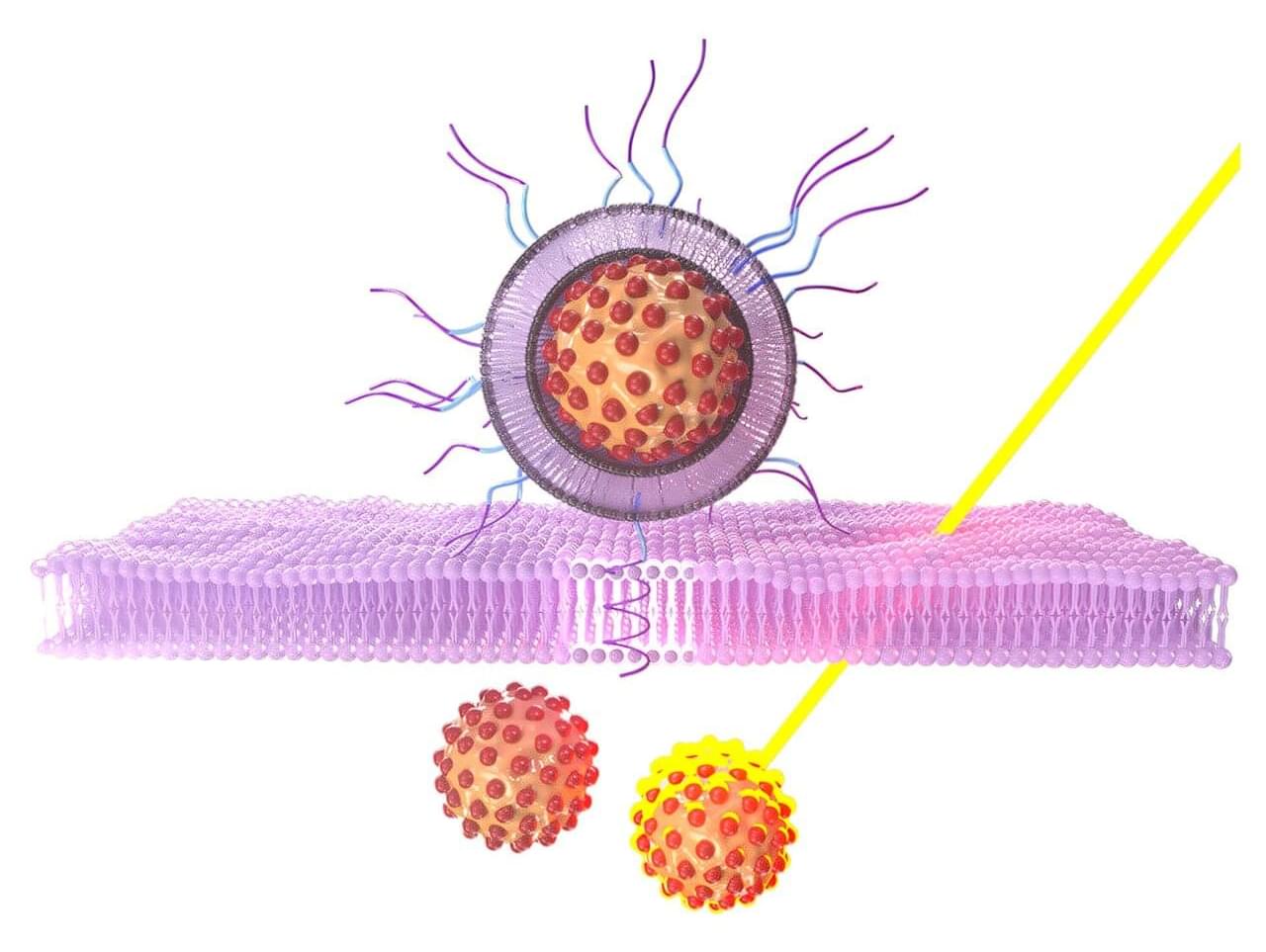
The NYU Abu Dhabi team designed tiny, biocompatible and biodegradable nanoparticles that carry a dye activated by near-infrared light. When exposed to this light, the particles heat up, damaging tumor tissue while minimizing harm to healthy cells. Near-infrared light was chosen specifically as it penetrates the body to greater depth than visible light, thereby enabling treatment of tumors that are not close to the surface.