An analysis of ongoing trials suggests that mRNA cancer vaccines have the potential to deliver health benefits worth $75 billion each year in the US alone


The modified strain grows faster, uses far fewer resources, and lowers greenhouse gas emissions by up to 60%. It also dramatically outperforms chicken farming in land and water use. The findings highlight a promising path for eco-friendly protein…
…’There is a popular demand for better and more sustainable protein for food,’ says corresponding author Xiao Liu of Jiangnan University in Wuxi, China. ‘We successfully made a fungus not only more nutritious but also more environmentally friendly by tweaking its genes.’
Animal agriculture contributes roughly 14% of global greenhouse gas emissions. It also requires large areas of land and significant amounts of fresh water, which are already under pressure from climate change and human activity. Because of these drawbacks, microbial proteins found in organisms such as yeast and fungi are gaining attention as a more sustainable alternative to traditional meat.
CRISPR has turned a simple fungus into a fast-growing, meat-like protein source with impressively low environmental impact.

Although we’ve heard a lot about how 3D-printing concrete homes speeds up the construction process, you still have to wait up to 28 days for the concrete to sufficiently cure. A new printable substitute, however, is ready to go in just three days.
Concrete consists of three parts: water, an aggregate such as sand or gravel, and a cement which binds everything together. The cement is the part that typically takes about a month to cure after being poured. And a slow curing time isn’t cement’s only problem.
Traditional Portland-style cement is made by grinding up limestone and other raw materials, then heating the resulting powder to temperatures of up to 1,450 ºC (2,642 ºF). Unfortunately, the processes by which that heat is generated produce a lot of carbon dioxide.

The first recipient of the Neuralink implant has successfully turned the X comment section wholesome after posting how the implant gave him a newfound sense of purpose.

Wearable electronics could be more wearable, according to a research team at Penn State. The researchers have developed a scalable, versatile approach to designing and fabricating wireless, internet-enabled electronic systems that can better adapt to 3D surfaces, like the human body or common household items, paving the path for more precise health monitoring or household automation, such as a smart recliner that can monitor and correct poor sitting habits to improve circulation and prevent long-term problems.
The method, detailed in Science Advances, involves printing liquid metal patterns onto heat-shrinkable polymer substrates—otherwise known as the common childhood craft “Shrinky Dinks.” According to team lead Huanyu “Larry” Cheng, James L. Henderson, Jr. Memorial Associate Professor of Engineering Science and Mechanics in the College of Engineering, the potentially low-cost way to create customizable, shape-conforming electronics that can connect to the internet could make the broad applications of such devices more accessible.
“We see significant potential for this approach in biomedical uses or wearable technologies,” Cheng said, noting that the field is projected to reach $186.14 billion by 2030. “However, one significant barrier for the sector is finding a way to manufacture an easy-to-customize device that can be applied to freestanding, freeform surfaces and communicate wirelessly. Our method solves that.”

Artificial light-type plant factories are an emerging agricultural innovation that enables crops to be grown year-round in precisely controlled environments. By adjusting factors such as light, temperature, humidity, carbon dioxide concentration, and nutrient delivery, these facilities can produce stable yields independent of climate conditions. They offer a promising way to reduce pesticide use and minimize the impacts of climate change.
However, legumes like edamame have long been considered difficult to cultivate in such settings because of their long growth periods, short storage periods, complex flowering, and pod-setting processes.
Against this backdrop, the research group, led by Professor Toshio Sano from the Faculty of Bioscience and Applied Chemistry, Hosei University, Japan, and Associate Professor Wataru Yamori of the Graduate School of Agricultural and Life Sciences, The University of Tokyo, Japan, had previously gained attention for successfully cultivating tomatoes under LED lighting in a plant factory.
It’s bound to happen at a summer picnic, a peaceful walk in the woods or simply sitting in your backyard… a mosquito targets your blood for its next meal. You’ve been bitten. But how do mosquitoes find you?
Among several methods used to locate new hosts for blood-sucking, mosquitoes feature a keen ability to detect carbon dioxide. As we breathe out, we emit CO2 into the air around us, which mosquitoes can sense. But how?
Scientists have been aware of the mosquito’s ability to detect our carbon dioxide expirations but the intricate underlying physiological structures enabling these capabilities have largely remained unclear.
Glioblastoma is one of the deadliest brain cancers, with a median survival rate of just 15 months. Despite surgery and chemotherapy, more than 1250 clinical trials over the past 20 years have struggled to improve survival rates.
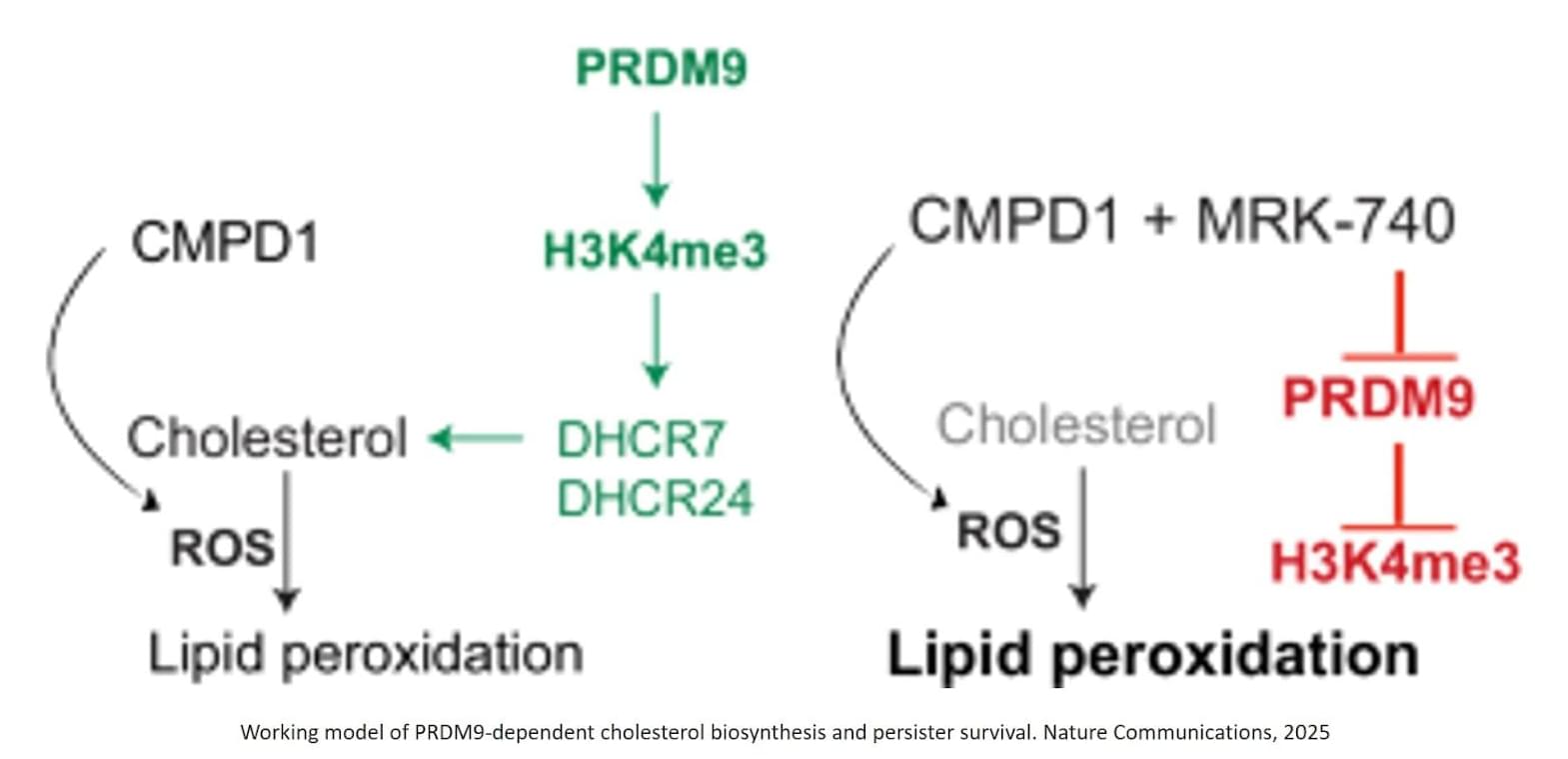
Published in Nature Communications, the study shows that a small population of drug-tolerant cells known as “persister cells” rewires its metabolism to survive chemotherapy, using an unexpected ally as an invisibility cloak: a fertility gene called PRDM9.
The authors identified that chemotherapy-induced PRDM9 upregulation promotes metabolic rewiring in glioblastoma stem cells, leading to chemotherapy tolerance. Mechanistically, PRDM9-dependent H3K4me3 at cholesterol biosynthesis genes enhances cholesterol biosynthesis, which persister cells rely on to maintain homeostasis under chemotherapy-induced oxidative stress and lipid peroxidation.
PRDM9 inhibition, combined with chemotherapy, results in strong anti-cancer efficacy in preclinical glioblastoma models, significantly enhancing the magnitude and duration of the antitumor response by eliminating persisters.
Previously, PRDM9 was only known as a fertility gene, active in reproductive cells at the very start of egg and sperm formation, long before fertilisation.
The researchers are now working with Australian biotech company Syntara to develop PRDM9 inhibitors for further testing in animal models, with the hope to eventually progress to human studies.