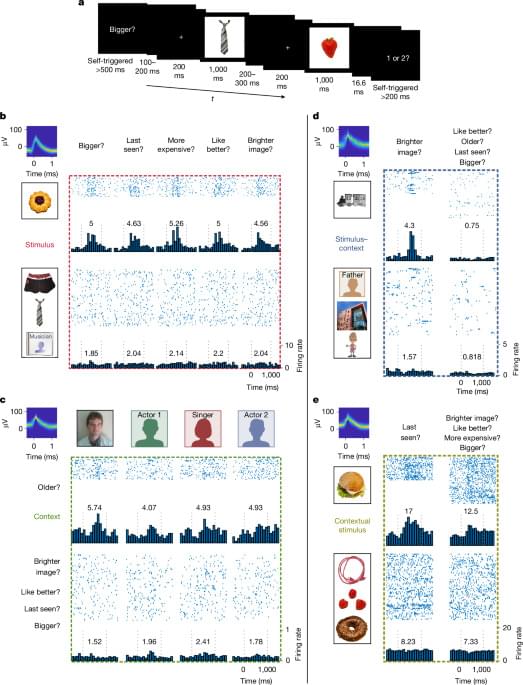
A new study shows that the human brain stores what we remember and the context in which it happens using different neurons.



Long-term exposure to fine particulate matter like PM2.5 components in polluted air can not only cause respiratory diseases, but also increase the risk of depression in older people, especially in those living with preexisting heart, metabolic and neurological conditions.
Depression has caused more loss of healthy life worldwide than any other mental health condition. This disorder has snatched away people’s will to perform the basics of daily activities. An analysis of global health data in 2021 showed that all the years people lived with disability or reduced quality of life because of depression added up to about 56.3 million years.
A recent population-based cohort study collected data from nearly 23.7 million U.S. Medicare beneficiaries aged 65 years and older between 2000 and 2018 to examine specific components of PM2.5 exposure, both individually and in combination, and its associations with the risk of developing depression. Among those tracked, more than 5.5 million developed depression during the follow-up period. These findings are published in JAMA Network Open.

The first recipient of the Neuralink implant has successfully turned the X comment section wholesome after posting how the implant gave him a newfound sense of purpose.
It’s bound to happen at a summer picnic, a peaceful walk in the woods or simply sitting in your backyard… a mosquito targets your blood for its next meal. You’ve been bitten. But how do mosquitoes find you?
Among several methods used to locate new hosts for blood-sucking, mosquitoes feature a keen ability to detect carbon dioxide. As we breathe out, we emit CO2 into the air around us, which mosquitoes can sense. But how?
Scientists have been aware of the mosquito’s ability to detect our carbon dioxide expirations but the intricate underlying physiological structures enabling these capabilities have largely remained unclear.
Glioblastoma is one of the deadliest brain cancers, with a median survival rate of just 15 months. Despite surgery and chemotherapy, more than 1250 clinical trials over the past 20 years have struggled to improve survival rates.
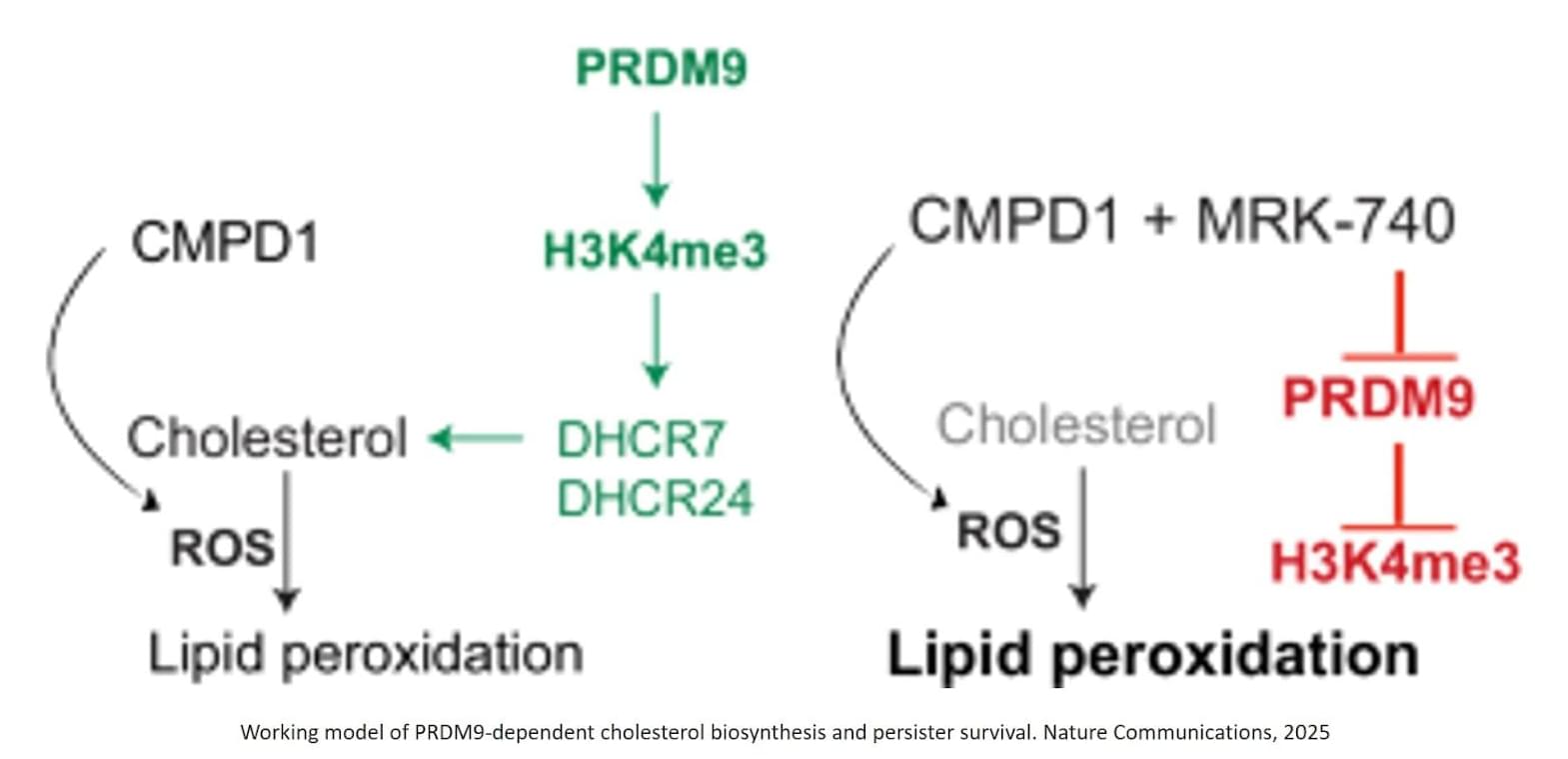
Published in Nature Communications, the study shows that a small population of drug-tolerant cells known as “persister cells” rewires its metabolism to survive chemotherapy, using an unexpected ally as an invisibility cloak: a fertility gene called PRDM9.
The authors identified that chemotherapy-induced PRDM9 upregulation promotes metabolic rewiring in glioblastoma stem cells, leading to chemotherapy tolerance. Mechanistically, PRDM9-dependent H3K4me3 at cholesterol biosynthesis genes enhances cholesterol biosynthesis, which persister cells rely on to maintain homeostasis under chemotherapy-induced oxidative stress and lipid peroxidation.
PRDM9 inhibition, combined with chemotherapy, results in strong anti-cancer efficacy in preclinical glioblastoma models, significantly enhancing the magnitude and duration of the antitumor response by eliminating persisters.
Previously, PRDM9 was only known as a fertility gene, active in reproductive cells at the very start of egg and sperm formation, long before fertilisation.
The researchers are now working with Australian biotech company Syntara to develop PRDM9 inhibitors for further testing in animal models, with the hope to eventually progress to human studies.

The 2026 Timeline: AGI Arrival, Safety Concerns, Robotaxi Fleets & Hyperscaler Timelines ## The rapid advancement of AI and related technologies is expected to bring about a transformative turning point in human history by 2026, making traditional measures of economic growth, such as GDP, obsolete and requiring new metrics to track progress ## ## Questions to inspire discussion.
Measuring and Defining AGI
🤖 Q: How should we rigorously define and measure AGI capabilities? A: Use benchmarks to quantify specific capabilities rather than debating terminology, enabling clear communication about what AGI can actually do across multiple domains like marine biology, accounting, and art simultaneously.
🧠 Q: What makes AGI fundamentally different from human intelligence? A: AGI represents a complementary, orthogonal form of intelligence to human intelligence, not replicative, with potential to find cross-domain insights by combining expertise across fields humans typically can’t master simultaneously.
📊 Q: How can we measure AI self-awareness and moral status? A: Apply personhood benchmarks that quantify AI models’ self-awareness and requirements for moral treatment, with Opus 4.5 currently being state-of-the-art on these metrics for rigorous comparison across models.
AI Capabilities and Risks.