Cold plunge and sauna benefits: how hot–cold therapy boosts recovery and longevity


For 25 years, scientists at Northwestern Medicine have been studying individuals aged 80 and older—dubbed “SuperAgers”—to better understand what makes them tick.
These unique individuals, who show outstanding memory performance at a level consistent with individuals who are at least three decades younger, challenge the long-held belief that cognitive decline is an inevitable part of aging.
Over the quarter-century of research, the scientists have seen some notable lifestyle and personality differences between SuperAgers and those aging typically—such as being social and gregarious—but “it’s really what we’ve found in their brains that’s been so earth-shattering for us,” said Dr. Sandra Weintraub, a professor of psychiatry and behavioral sciences and neurology at Northwestern University Feinberg School of Medicine.



A paper coauthored by geneticist George Church has been retracted following an internal review at a university where several coauthors are based.
The article appeared in the Proceedings of the National Academy of Sciences in 2022. The work supports an anti-aging gene therapy developed by BioViva, a company for which Church serves as an adviser. The paper’s authors claim cytomegalovirus (CMV) can be a gene therapy vector for a treatment for “aging-associated decline” that can be inhaled or injected monthly.
The work has been cited 41 times, two of which are citations from corrections to the article, according to Clarivate’s Web of Science.
Aging infrastructure, soaring electricity demand, renewable integration, and climate risks are driving the largest US grid modernization effort in history.

The idea of taking blood from the young to rejuvenate the elderly is getting an increasing amount of attention from scientists, and a new study has shown how some of the youthful properties of our skin can be restored with this kind of blood swap.
A special 3D human skin model was set up in the lab by researchers, who then tested the effects of young blood serum on the skin cells. By itself, the serum had no effect, but when bone marrow cells were added to the experiment, anti-aging signals were detected in the skin.
It appears that the young blood serum interacts with the bone marrow cells in specific ways to roll back time in skin cells. The study was led by scientists from Beiersdorf AG, a skin care company in Germany, who say their findings have huge potential in helping us understand anti-aging mechanisms.
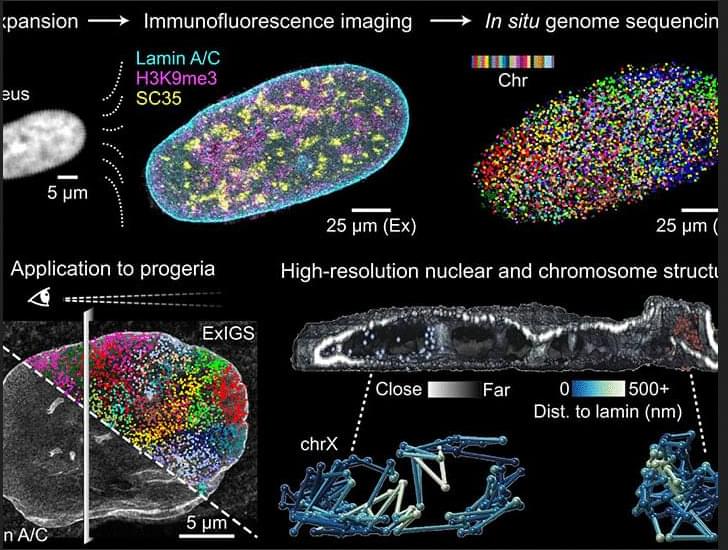
Great paper which combines expansion microscopy and in situ genome sequencing to map chromatin structure and selected protein targets in cellular nuclei. ExIGS was then used to explore how lamin protein and genome organization within nuclei changes during aging. #systemsbiology
Microscopy and genomics are used to characterize cell function, but approaches to connect the two types of information are lacking, particularly at subnuclear resolution. Here, we describe expansion in situ genome sequencing (ExIGS), a technology that enables sequencing of genomic DNA and super-resolution localization of nuclear proteins in single cells. Applying ExIGS to progeria-derived fibroblasts revealed that lamin abnormalities are linked to hotspots of aberrant chromatin regulation that may erode cell identity. Lamin was found to generally repress transcription, suggesting that variation in nuclear morphology may affect gene regulation across tissues and aged cells. These results demonstrate that ExIGS may serve as a generalizable platform with which to link nuclear abnormalities to gene regulation, offering insights into disease mechanisms.
Ovarian cancer is the leading cause of death among women with gynecological cancers. The current medical playbook—surgery followed by chemotherapy—initially shows promise. Tumors shrink, sometimes disappearing entirely. But in more than 80% of patients, the cancer not only comes back, but returns more aggressive and increasingly resistant to the very treatments that once seemed effective.
But now, there could be new hope. In a study published in the journal Med, UCLA researchers have detailed their development of a new type of immune cell therapy, called CAR-NKT cell therapy, that could transform ovarian cancer care by delivering superior cancer-fighting power.
“This is the culmination of over a decade of work in my lab and represents over six years of collaboration with gynecologic oncologist Dr. Sanaz Memarzadeh,” said co-senior author Lili Yang, a professor of microbiology, immunology and molecular genetics and a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA.
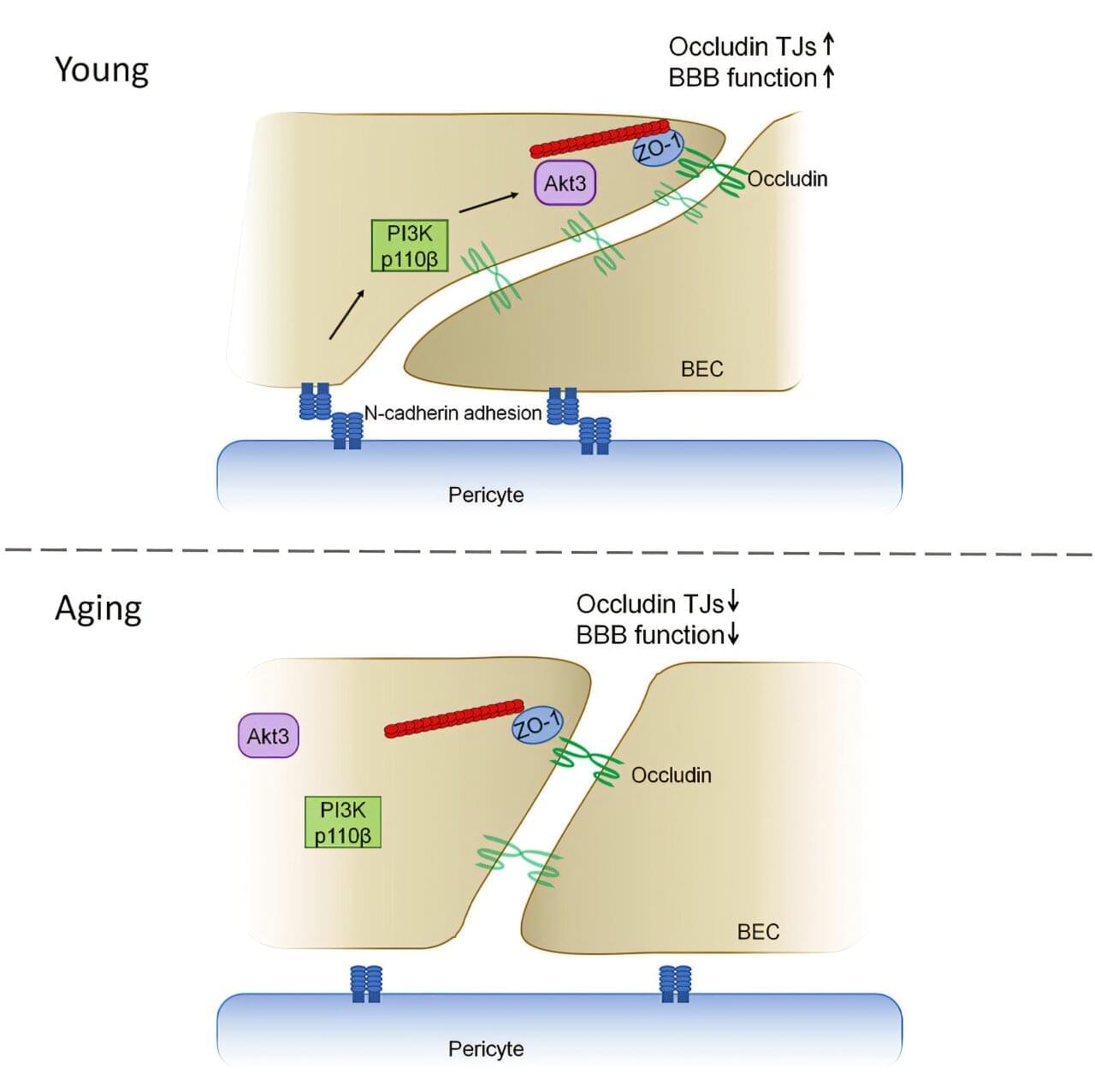
A new study from researchers at the University of Illinois Chicago reveals how the blood-brain barrier gets leakier with age, contributing to memory deficits. The study, published in Cell Reports, uncovered the molecular mechanisms behind this process and could provide new therapeutic targets to address cognitive decline earlier in the aging process.
The blood-brain barrier is a layer of cells lining the brain’s blood vessels that keep viruses, bacteria and toxins out while allowing helpful nutrients and chemicals in. A key structure of the blood-brain barrier are tight junctions that act as bridges between cells, restricting entry of molecules. A protein called occludin helps fulfill this essential role.
“It’s a highly regulatable process that allows some molecules to go through and others to remain in circulation,” said Yulia Komarova, UIC associate professor in the department of pharmacology and regenerative medicine at the College of Medicine and senior author of the study. “Basically, it’s a mechanism that separates the central nervous system from everything else.”