The research, published in Proceedings of the National Academy of Sciences, used a genetic approach to fix deafness in mice with a defective Spns2 gene, restoring their hearing abilities in low and middle frequency ranges. Researchers say this proof-of-concept study suggests that hearing impairment resulting from reduced gene activity may be reversible.
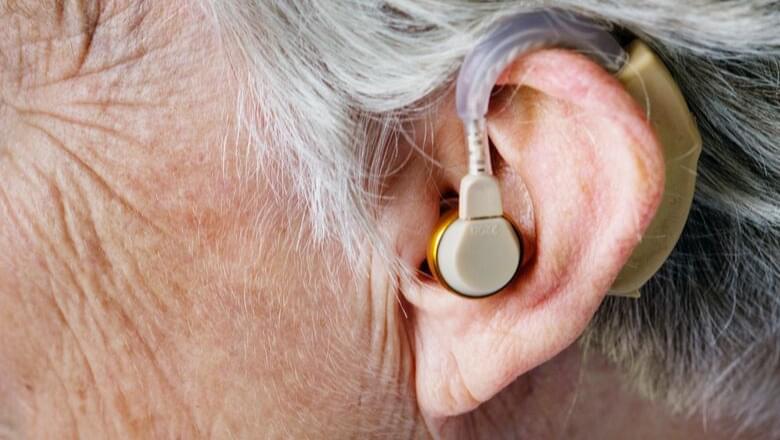
Over half of adults in their 70s experience significant hearing loss. Impaired hearing is associated with an increased likelihood of experiencing depression and cognitive decline, as well as being a major predictor of dementia. While hearing aids and cochlear implants may be useful, they do not restore normal hearing function, and neither do they halt disease progression in the ear. There is a significant unmet need for medical approaches that slow down or reverse hearing loss.
New research from the Institute of Psychiatry, Psychology & Neuroscience (IoPPN) at King’s College London has successfully reversed hearing loss in mice.
This proof-of-concept study suggests that gene therapy for this type of hearing loss in humans may be successful in the future.