Fresh from solving the protein structure challenge, Google’s deep-learning outfit is moving on to the human genome.


Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links:
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/
Use Code: ConquerAging At Checkout.
Oral Microbiome: https://www.bristlehealth.com/?ref=michaellustgarten.
Enter Code: ConquerAging.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Epigenetic Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
At-Home Blood Testing (SiPhox Health): https://getquantify.io/mlustgarten.
Studying genes in families with a propensity for certain diseases has led to many critical advances in medicine, including the discovery of statins in family members who suffered heart attacks at an early age.
Now, a team of researchers at Case Western Reserve University has identified an inherited mutation in a gene linked to a highly lethal cancer called esophageal adenocarcinoma (EAC).
“With this discovery, we will be able to identify early those at a high risk of developing EAC in their lifetime, and accordingly tailor screening, lifestyle and treatment strategies to prevent cancer development,” said Kishore Guda, an associate professor at the Case Western Reserve School of Medicine and member of the Case Comprehensive Cancer Center.

New research indicates that butterflies and moths share “blocks” of DNA
DNA, or deoxyribonucleic acid, is a molecule composed of two long strands of nucleotides that coil around each other to form a double helix. It is the hereditary material in humans and almost all other organisms that carries genetic instructions for development, functioning, growth, and reproduction. Nearly every cell in a person’s body has the same DNA. Most DNA is located in the cell nucleus (where it is called nuclear DNA), but a small amount of DNA can also be found in the mitochondria (where it is called mitochondrial DNA or mtDNA).
New research provides insight about the bedrock scientific principle that mitochondrial DNA -; the distinct genetic code embedded in the organelle that serves as the powerplant of every cell in the body -; is exclusively passed down by the mother.
The study, a collaboration among Oregon Health & Science University and other institutions, published today in the journal Nature Genetics.
Scientists have long recognized the fact that mitochondrial DNA, or mtDNA, comes exclusively from egg cells in humans, meaning only the mother contributes the genetic code carried by thousands of mitochondria necessary for energy production in every cell in the body.
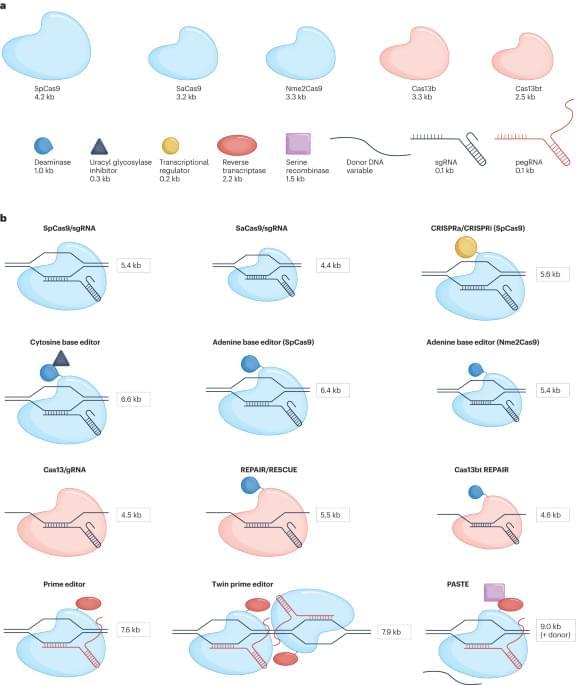
CRISPR-based genome editing has the potential to treat many human genetic diseases, but achieving stable, efficient and safe in vivo delivery remains a challenge. This Review assesses current delivery systems for genome editors—focusing on adeno-associated viruses and lipid nanoparticles—and highlights data from recent clinical trials. Emerging delivery systems and ongoing challenges in the field are discussed.

Summary: Researchers pioneered a groundbreaking method called “CHOOSE” to investigate genes tied to autism spectrum disorder (ASD) within human tissue. This technique allows for simultaneous examination of key transcriptional regulator genes linked to autism in a single organoid.
Utilizing CHOOSE, the team pinpointed mutations in 36 genes known to heighten autism risk, shedding light on how they influence brain development. The revelations from these organoids mirrored clinical observations, underscoring the potential of this method in advancing our understanding of neurodevelopmental disorders.
Year 2023 😗
Data from patients with AMD, retinal dystrophies, and diabetic retinopathy indicate an important role of immune cells, including microglia, in the pathogenesis of these retinal diseases1. The accumulation of drusen components provides an environment rich in chemoattractants for microglia and leads to their translocation to the subretinal space in AMD2,4. The involvement of microglia in the activation of the NLRP3 inflammasome and the promotion of proinflammatory cytokine secretion has been confirmed in in vitro and animal studies11,12,14. In patients with retinal dystrophies like retinitis pigmentosa, it has been shown that microglia become activated in response to signals from degenerating rod photoreceptors and migrate to the outer retinal layers4. There, they participate in the phagocytosis of debris and dying cells and secrete proinflammatory factors. Mouse models of retinal degeneration (e.g. rd1, rd7, rd8, and rd10 models) confirm many of these conclusions9,10,13,15, but make it clear that the role of microglia may also be homeostatic, depending on both stimuli and anatomical location within the retina7,20. Activated microglia are observed at all the stages of human diabetic retinopathy3,8 and also feature prominently in many animal models of the disease44,45. Finally, accumulations of activated microglia are also seen in a variety of animal models of retinal degeneration, including light-induced retinal degeneration and models based on complement dysregulation34,46,47.
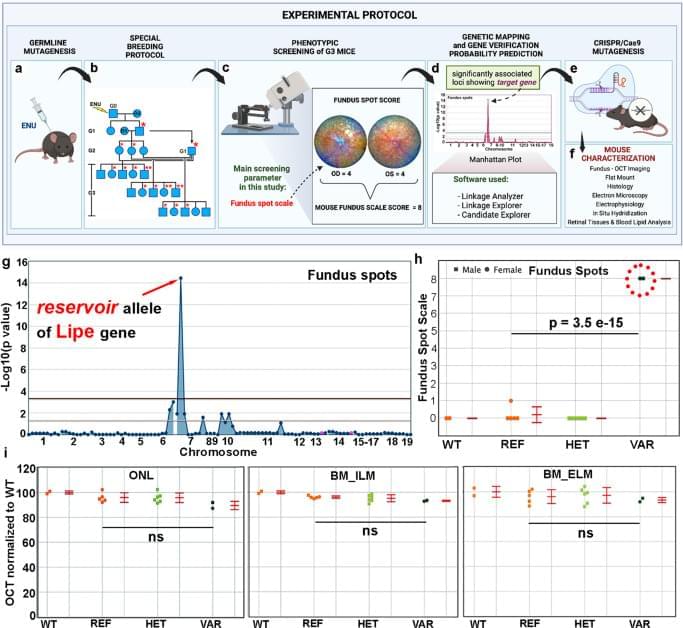
The pathways regulating immune surveillance, cell trafficking, and neuroinflammation in the retina are not well understood. A large number of molecules and processes have been implicated, ranging from chemokines involved in chemotaxis, cytokines involved in activation, factors that regulate oxidative stress and complement activation, and immunoregulatory proteins. In such a complex biological system, the unbiased nature of a forward genetics approach is particularly valuable in identifying genes affecting these immune cell processes. Furthermore, the accumulation of subretinal microglia, visible as or correlated with the accumulation of fundus spots, can serve as a marker for retinal pathology and thus as a screen for genes essential to retinal homeostasis. Our approach here has two important advantages relative to all prior forward genetics studies of the retina: 1. We are systematically applying a semiquantitative fundus spot scale to fundus photographs, and 2. Our pipeline is the only one in which all mice screened are G3 mice that have been pre-genotyped at all mutant loci. Our unbiased identification of 6 gene-phenotype associations to retinal pathology with strong literature support using our fundus spot scale screen is proof of concept supporting the efficacy of our approach. We identified other associations that had not been reported in the literature at the time of the screening. From those, we first concentrated our efforts on the gene Lipe, partly because the fundus spot scale was the only parameter leading to its identification.
In order to confirm our findings in ENU-mutagenized mice and also to explore the role of Lipe in retinal homeostasis, a CRISPR-generated Lipe−/− mouse line was generated. Imaging of the retinas on these mice confirmed an early and prominent accumulation of fundus spots. Furthermore, we found a similar widespread accumulation of hyperautofluorescent spots in these mice. We were also able to show that Lipe−/− mice have increased accumulation of subretinal Iba1+/CD16+/TMEM119+/CCR2− cells consistent with activated microglia. It can be argued that microglia migrating to the subretinal space are by definition showing some level of activation48,49,50. But our findings of well-accepted morphological signs of activation and co-staining with CD16, a marker of microglial activation10,34,51,52, further support this conclusion.