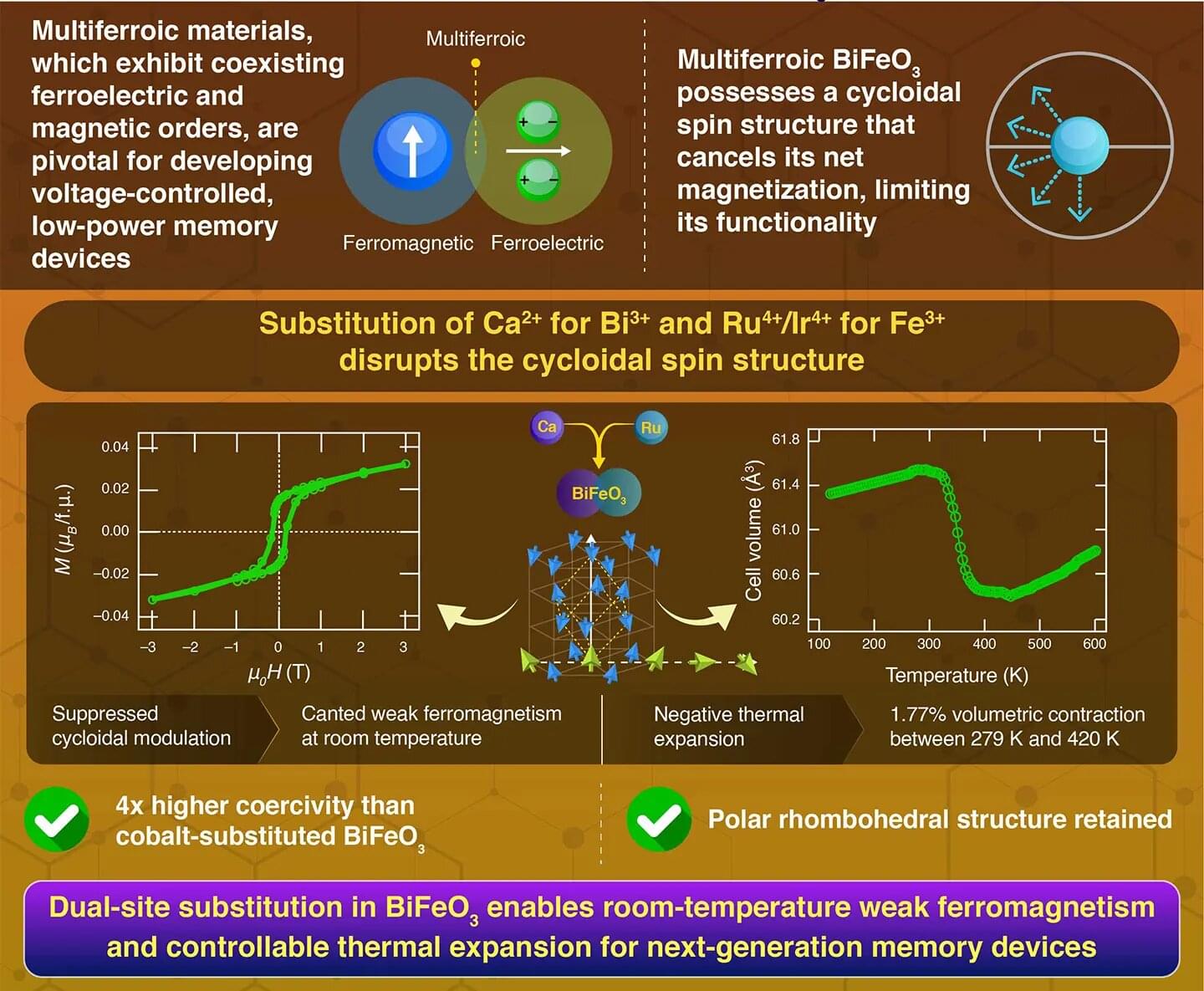
Carbon capture is becoming essential for industries that still depend on fossil fuels, including the cement and steel industries. Natural-gas power plants, coal plants, and cement factories all release large amounts of CO₂, and reducing those emissions is difficult without dedicated capture systems. Today, most plants rely on solvent-based systems that absorb CO₂, but these setups use a lot of heat, require major infrastructure, and can be costly to run.
A smaller, electricity-driven alternative is what the field calls a “membrane” system. A membrane works like an ultra-fine filter that lets certain gases slip through more easily than others, separating CO₂ from the rest of the flue gas. The problem is that many membranes lose efficiency when CO₂ levels are low, which is common in natural-gas plants, and this limits where they can be used.
A new study at EPFL has now analyzed how a new membrane material, pyridinic-graphene, could work at scale. This is a single-layer graphene sheet with tiny pores that favor CO₂ over other gases. The researchers combined experimental performance data with modeling tools that simulate real operating conditions, such as energy use and gas flow. They also explored a wide range of cost scenarios to see how the material might behave once deployed in commercial plants.