Amazon reports a GRU-linked APT44 campaign from 2021–2025 targeting energy and critical infrastructure using misconfigured network edge devices.


But the report was also marked by the flaws of its technocratic conception. Crucially, the broader stakes of the global energy transition went ignored. This is a mistake in urgent need of correction. The decarbonization agenda is not simply about reordering markets or industrial policies, but in fact represents the crucible for a new geopolitical order.
Four years ago, the International Energy Agency (IEA) published a landmark report, “Net Zero by 2050: A Roadmap for the Global Energy Sector,” that proposed a technical blueprint for a global green energy transition by the middle of this century. The report focused on the economic and technological dimensions of this energy transition. It was an admirable effort that calls for careful study.
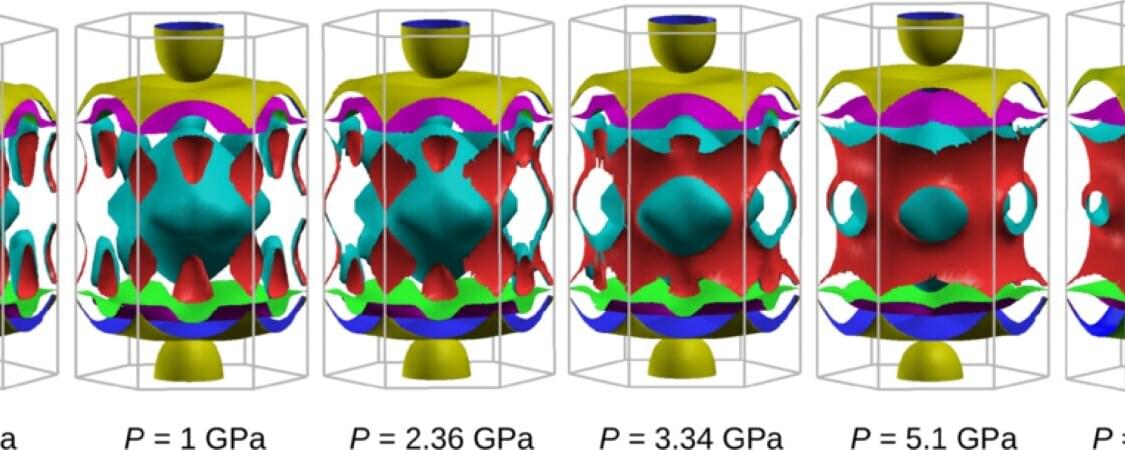
Scientists have discovered a way to efficiently transfer electrical current through specific materials at room temperature, a finding that could revolutionize superconductivity and reshape energy preservation and generation.
The paper is published in the journal Physical Review Letters.
The much-sought-after breakthrough hinges on applying high pressure to certain materials, forcing their electrons closer together and unlocking extraordinary electronic behaviors.

Carbon capture is becoming essential for industries that still depend on fossil fuels, including the cement and steel industries. Natural-gas power plants, coal plants, and cement factories all release large amounts of CO₂, and reducing those emissions is difficult without dedicated capture systems. Today, most plants rely on solvent-based systems that absorb CO₂, but these setups use a lot of heat, require major infrastructure, and can be costly to run.
A smaller, electricity-driven alternative is what the field calls a “membrane” system. A membrane works like an ultra-fine filter that lets certain gases slip through more easily than others, separating CO₂ from the rest of the flue gas. The problem is that many membranes lose efficiency when CO₂ levels are low, which is common in natural-gas plants, and this limits where they can be used.
A new study at EPFL has now analyzed how a new membrane material, pyridinic-graphene, could work at scale. This is a single-layer graphene sheet with tiny pores that favor CO₂ over other gases. The researchers combined experimental performance data with modeling tools that simulate real operating conditions, such as energy use and gas flow. They also explored a wide range of cost scenarios to see how the material might behave once deployed in commercial plants.
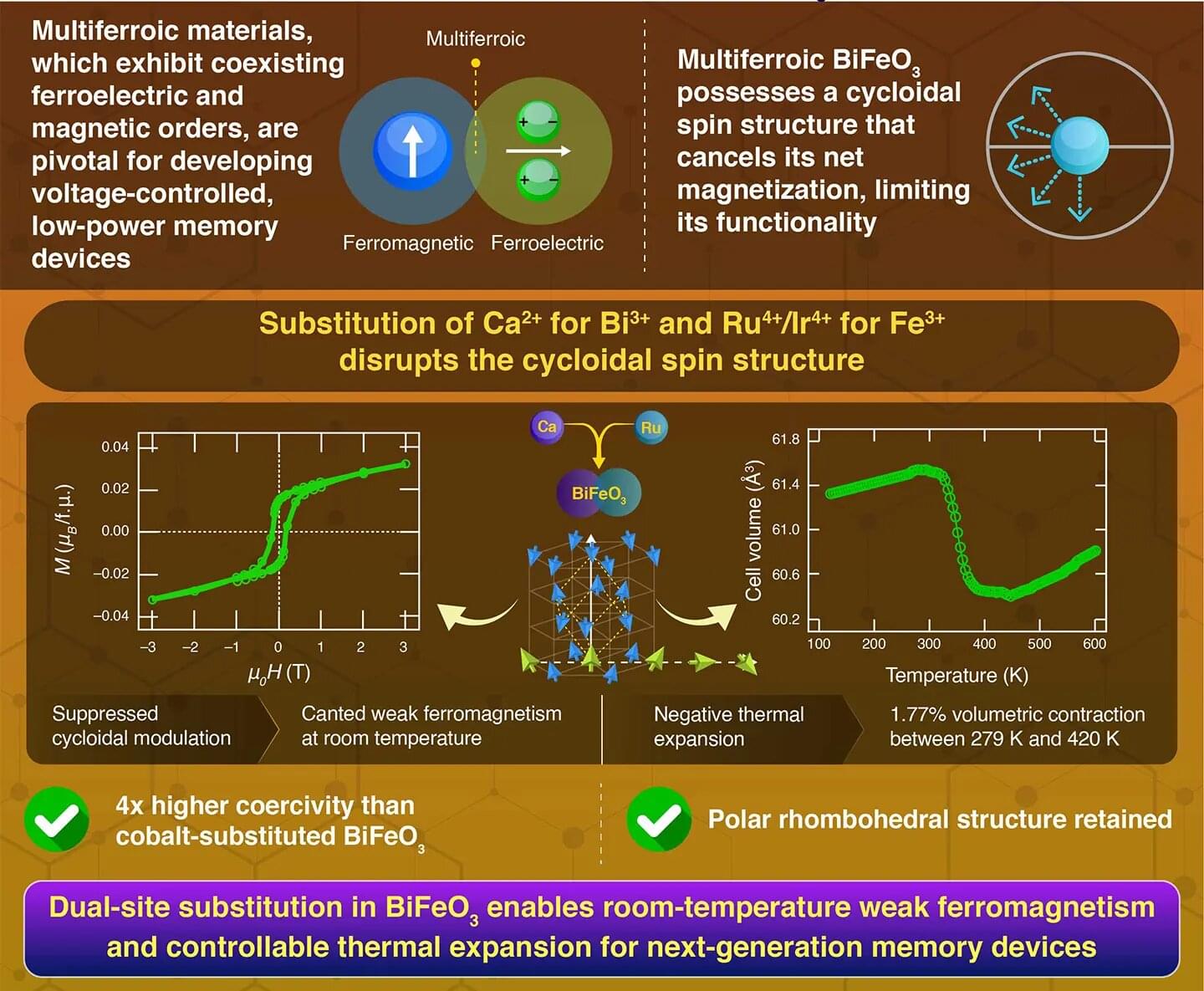
Using a dual-cation substitution approach, researchers at Science Tokyo introduced ferromagnetism into bismuth ferrite, a well-known and promising multiferroic material for next-generation memory technologies. By replacing ions at both the bismuth and iron sites with calcium ions and heavier elements, they modified the spin structure and achieved ferromagnetism at room temperature. Additionally, negative thermal expansion was observed. This ability to engineer magnetism and thermal expansion in a multiferroic material aids in realizing future memory devices.
Multiferroic materials, which show both ferroelectricity and ferromagnetism, hold strong potential for use in low-power memory devices where information can be written electrically and read magnetically. Among these materials, bismuth ferrite (BiFeO3) is one of the most widely studied because it combines ferroelectricity with antiferromagnetism at room temperature.
However, BiFeO3 naturally forms a cycloidal spin structure, which is a wave-like pattern of rotating spins. This pattern cancels out any net magnetization and makes the material difficult to use in magnetic devices.

Running a synchrotron light source is a massive team effort that brings hundreds of highly skilled and specialized professionals together. The radiofrequency (RF) group at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Brookhaven National Laboratory, plays an integral role in synchrotron operations. The work they do, often behind the scenes, ensures that the electron beam that enables cutting-edge science at NSLS-II remains bright, powerful, and stable.
The electrons that circle through NSLS-II’s nearly half-mile-long storage ring lose energy as they produce X-rays, which scientists use to perform a variety of experiments at the facility. To keep the beam moving steadily, the electrons pass through hollow RF cavities. These cavities, tuned to a precise frequency, restore the electrons’ energy each time they pass through.
When cooled to cryogenic temperatures, the material that the cavities are comprised of, niobium, takes on superconducting properties that nearly eliminate electrical resistance and drastically improve energy efficiency and beam stability. The design also allows unwanted high-frequency oscillations to be safely damped, ensuring a stable, high-intensity X-ray beam.

Online discussions are often dominated by a small group of active users, while the majority remain silent. This imbalance can distort perceptions of public opinion and fuel polarization.
In a group-based field experiment on Reddit, researchers from the Max Planck Institute for Human Development, TU Dresden, and Stanford University have investigated why some people remain silent readers (“lurkers”) while others are particularly active (“power users”)—and which measures might encourage people to join the discussion.
Results from the experiment are published in the journal Science Advances.

The full structure, if it is ever built, will likely take centuries. But the first pieces could be deployed within the next hundred years. Like the construction of cathedrals or transcontinental railroads, this is the kind of project that begins with a vision and takes shape slowly, across generations.
A Dyson sphere is not only about energy. It is about how we think. It challenges us to plan at the scale of a civilization rather than a single generation. It asks what kind of future we are trying to build, and whether we are willing to imagine a world of abundance rather than scarcity. It may take centuries to complete, but the mindset that makes it possible can start right now.