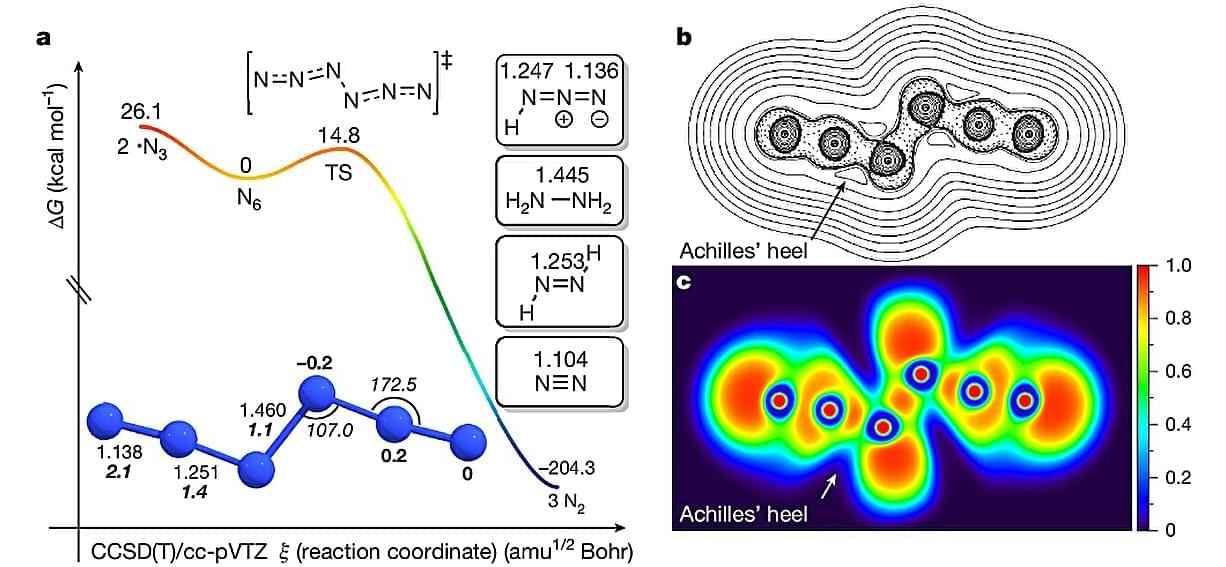
Nitrogen finally joins the elite tier of elements like carbon that can form neutral allotropes—different structural forms of a single chemical element. Researchers from Justus Liebig University, Giessen, Germany, have synthesized neutral hexanitrogen (N6)—the first neutral allotrope of nitrogen since the discovery of naturally occurring dinitrogen (N2) in the 18th century that is cryogenically stable and can be prepared at room temperature.
This new study, published in Nature, synthesized hexanitrogen (N6) via gas-phase reaction, with the main ingredients being chlorine (Cl2) or bromine (Br2) and an extremely reactive and explosive solid silver azide (AgN3), under reduced pressure.
The researchers spread AgN3 on the inner surface, and a gaseous halogen (Cl2 or Br2) was passed through the solid under reduced pressure at room temperature. The reaction triggered by the process produced N6 alongside byproducts chloronitrene (ClN) and hydrazoic acid (HN3).