A lot of information to see your brains complexity.
Mind-boggling mind research.
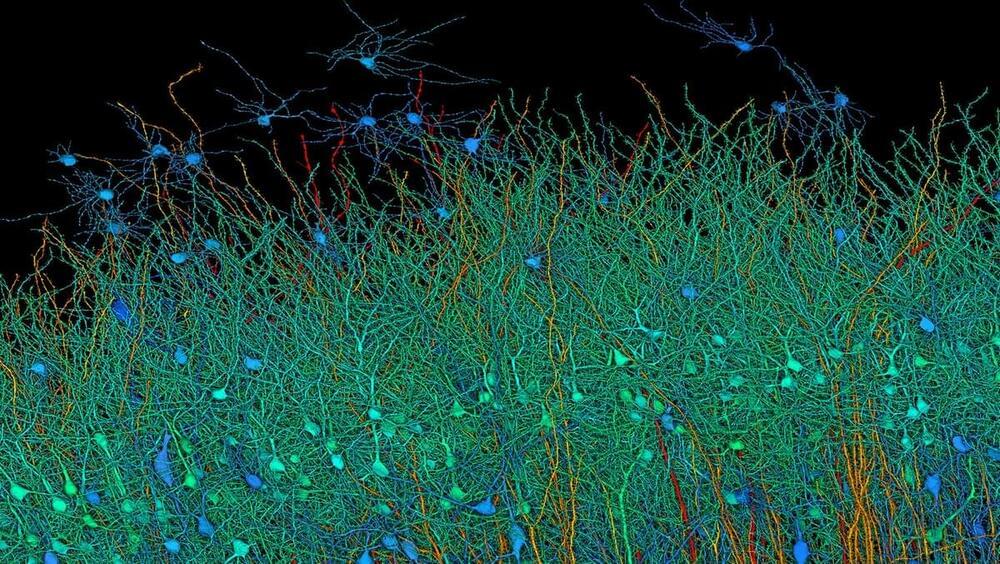

ALGORITHMS THAT DECODE IMAGES A PERSON SEES OR IMAGINES will enable visual representations of dreams a sleeper is having, and give deeper insights into emotionally disturbed or mentally ill patients.
Go to a href= https://brilliant.org/coldfusion
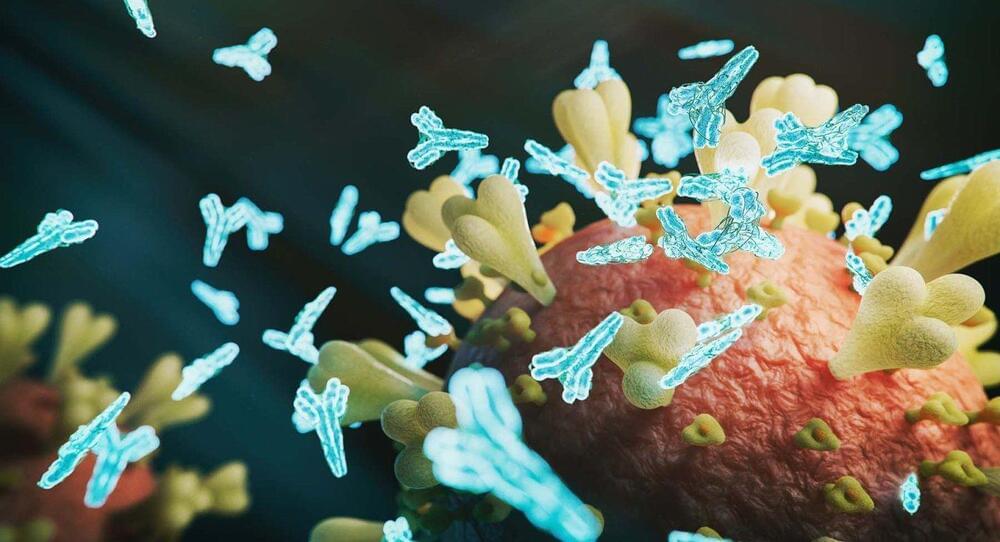
In a discovery that could hasten treatment for patients with multiple sclerosis (MS), UC San Francisco scientists have discovered a harbinger in the blood of some people who later went on to develop the disease.
In about 1 in 10 cases of MS, the body begins producing a distinctive set of antibodies against its own proteins years before symptoms emerge. These autoantibodies appear to bind to both human cells and common pathogens, possibly explaining the immune attacks on the brain and spinal cord that are the hallmark of MS.
The findings were published in Nature Medicine on April 19.
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhDDiscount Links: Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7x…


Sometimes when you’re considering how to bring the power of AI to a clinical context, it sort of takes a new way of thinking to get inspired about what’s possible.
I was thinking about this the other day, inspired by some people who have been working hard on genomics, oncology research, and other types of biological and anatomical applications. There’s so much of it, suddenly, especially at these institutions that I’m so close to – to call it a “revolution” in my view, isn’t hyperbolic.
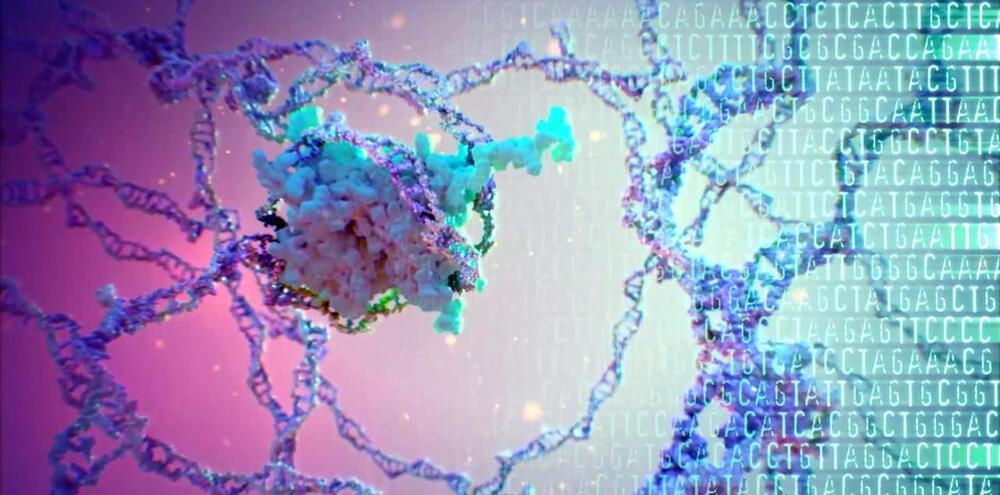
The brain is one of the most complex entities in biology. For thousands of years, humans have wondered how the human brain works, but only in the past few years has technology evolved so that scientists can actually answer some of the many questions we have. What are the causes of brain disorders? How do our brains develop? How does the brain heal after a head injury? While we still have a long way to go before we can understand the many facets of the human brain, one technology – CRISPR – has allowed us to start answering these questions on a genetic level.
What is CRISPR?

What if someone handed you a tool and said that you could better the lives of people before their birth by changing their genes? Would you do it?
CRISPR-Cas9 is one such tool. It’s an efficient and effective gene-editing technology that works by tagging a section of DNA with an RNA segment, and then using a protein called Cas9 to cut the DNA at the specified point. Then, the cell’s own DNA machinery works to add or delete DNA.
This technology opens up the pathway to a variety of gene-editing applications, from eliminating HIV in living organisms to creating a potential cure for Huntington’s disease. There is especially high potential for single-gene disorders to be eradicated. For example, promising results from the successful removal of a gene known to cause fatal heart disease from the embryo will not only save lives but also prevent the passing down of the gene.

Down syndrome (DS) is one of the most prevalent genetic disorders in humans. The use of new approaches in genetic engineering and nanotechnology methods in combination with natural cellular phenomenon can modify the disease in affected people. We consider two CRISPR/Cas9 systems to cut a specific region from short arm of the chromosome 21 (Chr21) and replace it with a novel designed DNA construct, containing the essential genes in chromatin remodeling for inactivating of an extra Chr21. This requires mimicking of the natural cellular pattern for inactivation of the extra X chromosome in females. By means of controlled dosage of an appropriate Nano-carrier (a surface engineered Poly D, L-lactide-co-glycolide (PLGA) for integrating the relevant construct in Trisomy21 brain cell culture media and then in DS mouse model, we would be able to evaluate the modification and the reduction of the active extra Chr21 and in turn reduce substantial adverse effects of the disease, like intellectual disabilities. The hypothesis and study seek new insights in Down syndrome modification.
Keywords: Down syndrome, CRISPR/Cas9, Designed DNA construct, Poly D L-lactide-co-glycolide (PLGA), Nano-carrier, Chromosome 21 inactivation.