Membrane protein PEAR1 prevents the development of metastases


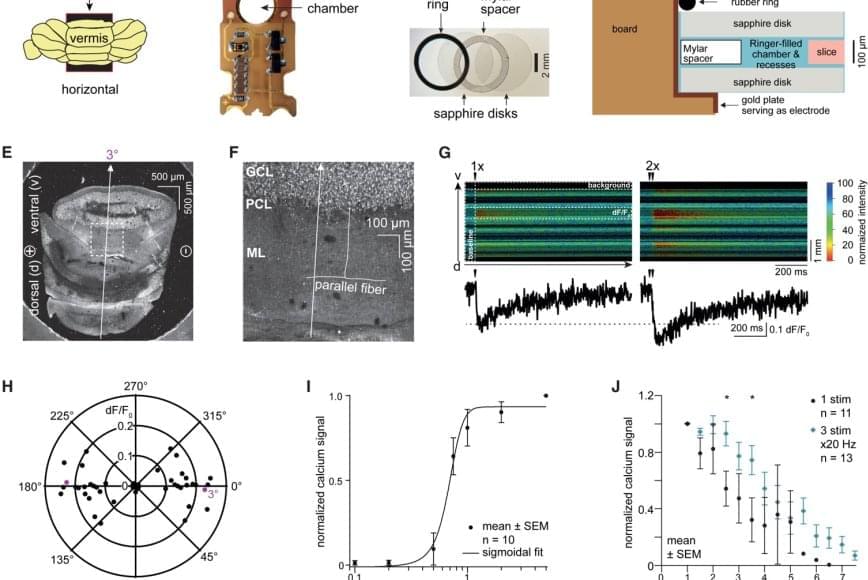
For the new study, the researchers used samples from the brains of normal mice as well as living cortical brain tissue sampled with permission from six individuals undergoing surgical treatment for epilepsy. The surgical procedures were medically necessary to remove lesions from the brain’s hippocampus.
The researchers first validated the zap-and-freeze approach by observing calcium signaling, a process that triggers neurons to release neurotransmitters in living mouse brain tissues.
Next, the scientists stimulated neurons in mouse brain tissue with the zap-and-freeze approach and observed where synaptic vesicles fuse with brain cell membranes and then release chemicals called neurotransmitters that reach other brain cells. The scientists then observed how mouse brain cells recycle synaptic vesicles after they are used for neuronal communication, a process known as endocytosis that allows material to be taken up by neurons.
The researchers then applied the zap-and-freeze technique to brain tissue samples from people with epilepsy, and observed the same synaptic vesicle recycling pathway operating in human neurons.
In both mouse and human brain samples, the protein Dynamin1xA, which is essential for ultrafast synaptic membrane recycling, was present where endocytosis is thought to occur on the membrane of the synapse.
“Our findings indicate that the molecular mechanism of ultrafast endocytosis is conserved between mice and human brain tissues,” the author says, suggesting that the investigations in these models are valuable for understanding human biology.


Cancer treatment with a cell-based immunotherapy causes mild cognitive impairment, a Stanford Medicine team found. They also identified compounds that could treat it.


In a patient from a histoplasmosis-endemic region, laryngeal histoplasmosis caused by Histoplasma capsulatum was diagnosed after biopsy revealed granulomatous inflammation and fungal organisms.
Early consideration and biopsy are key when evaluating ulcerative laryngeal lesions.
A 61-year-old man presented to the otolaryngology clinic with a 2-month history of progressive hoarseness, dysphagia, odynophagia, and persistent globus sensation. What is your diagnosis?

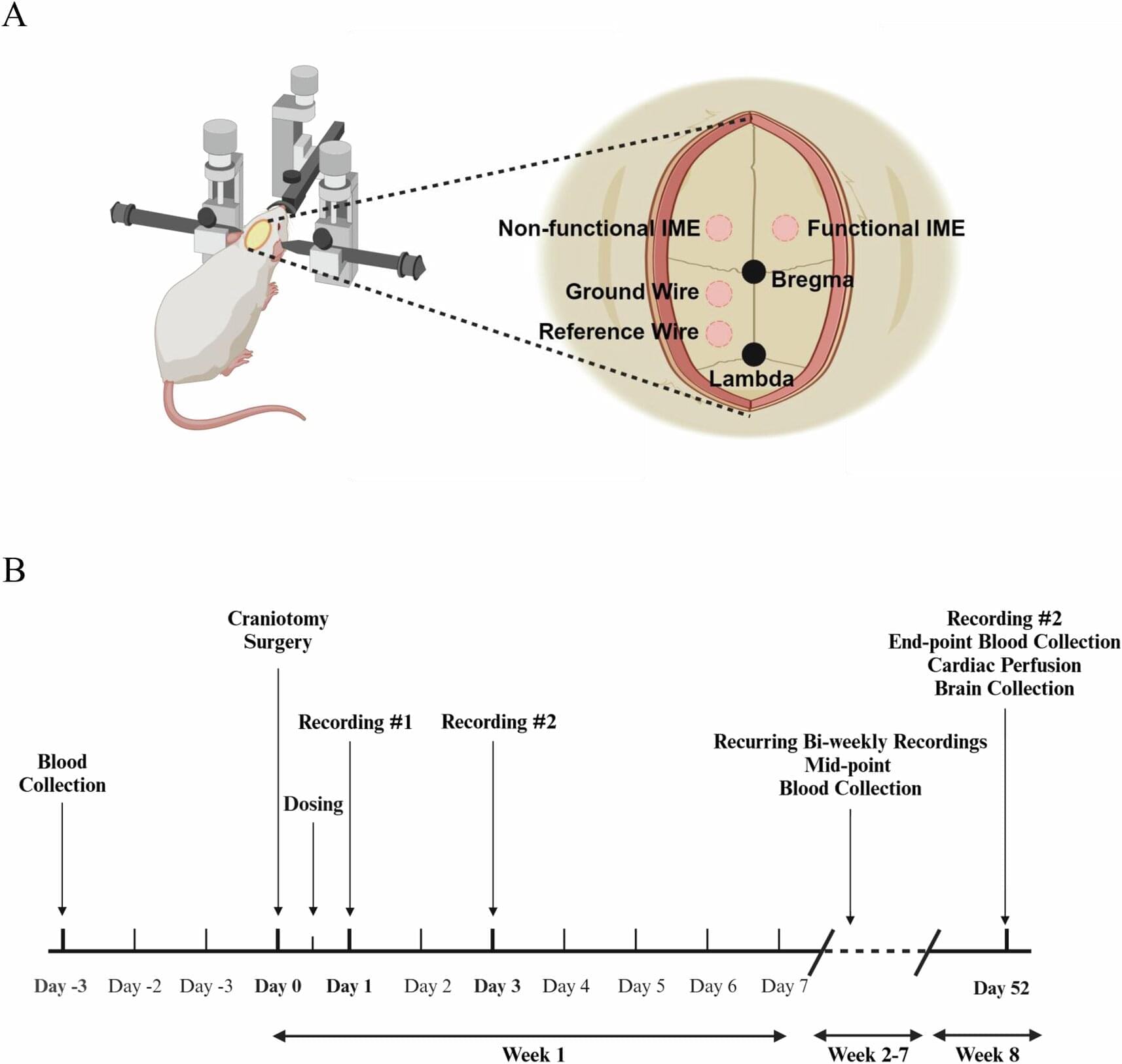
As part of the ESA Academy Experiments Programme, Team V-STARS carried out the first experiment with human participants in the Orbital Robotics Lab, investigating how microgravity affects the perception of verticality.
The V-STARS team, a collaboration between Birkbeck, University of London, and the University of Kent (UK), was selected to join the ESA Academy Experiments Programme in February 2025. After obtaining ethical approval from the United Kingdom and authorisation from the ESA Medical Board, the team was permitted to carry out their experiment in the Orbital Robotics Lab (ORL), located at ESTEC, the ESA site in the Netherlands.
The campaign involved test subjects seated on the ORL’s floating platform, wearing VR headsets while performing gravity-related perceptual tasks. The project investigates the use of Vestibular Stochastic Resonance — a phenomenon in which controlled noise enhances the sensitivity of a sensory system — to improve perception and potentially accelerate adaptation to microgravity. Over two weeks, the team tested more than 20 participants and has now returned to their universities to analyse the results.
Canine induced pluripotent stem (iPS) cells possess the ability to differentiate into any type of cell, making them a useful tool for investigating common canine diseases and disease states, including those of humans.
When culturing iPS cells, a culture substrate is required to serve as a scaffold for the cells, which adhere to it and proliferate. Without the scaffold, the cells die or fail to differentiate.
Currently, recombinant proteins derived primarily from humans are used as culture substrates for canine iPS cells. However, these human-derived elements are an alien substance for dog cells, leading to immune rejection and making clinical use difficult.