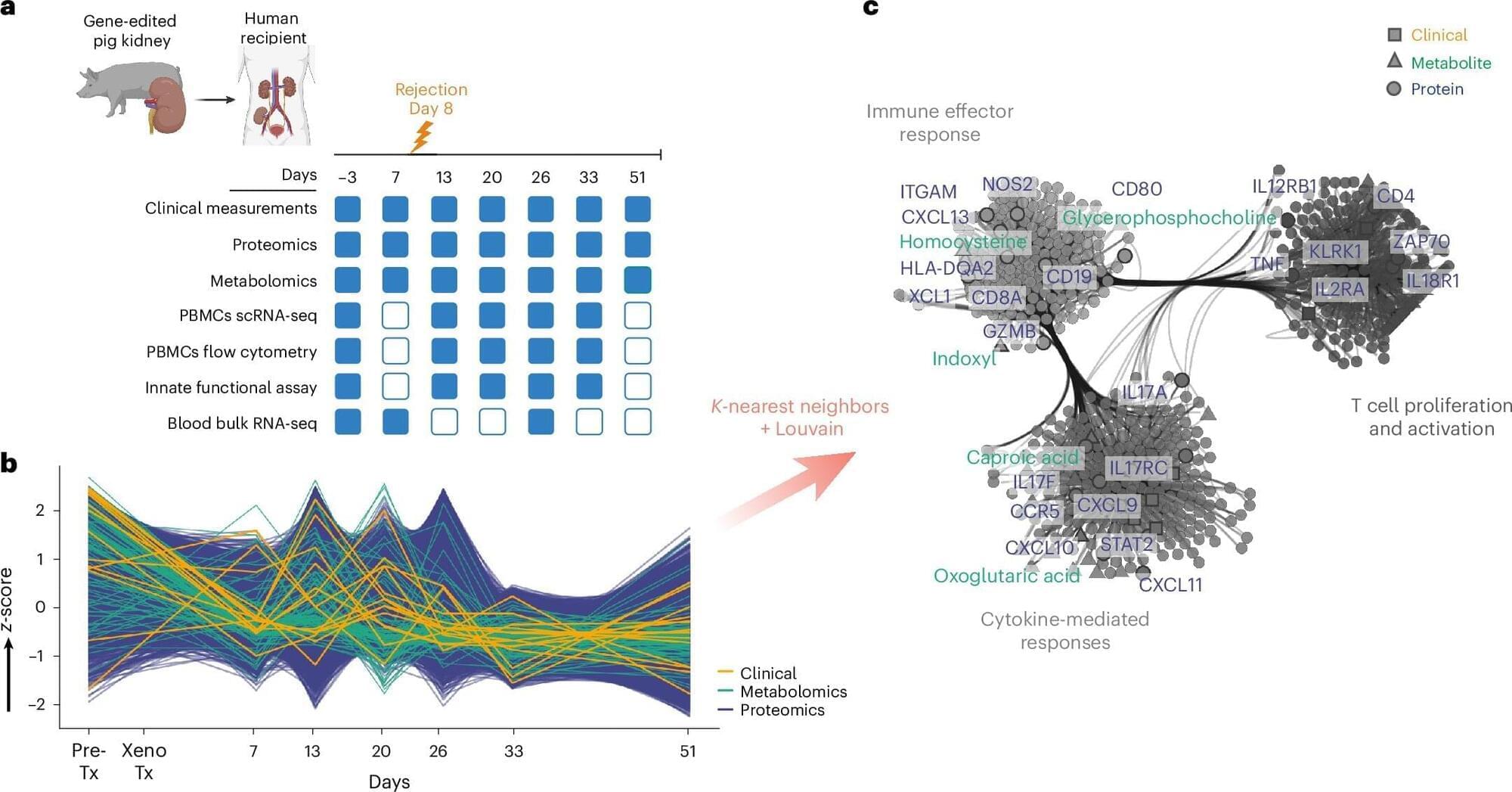
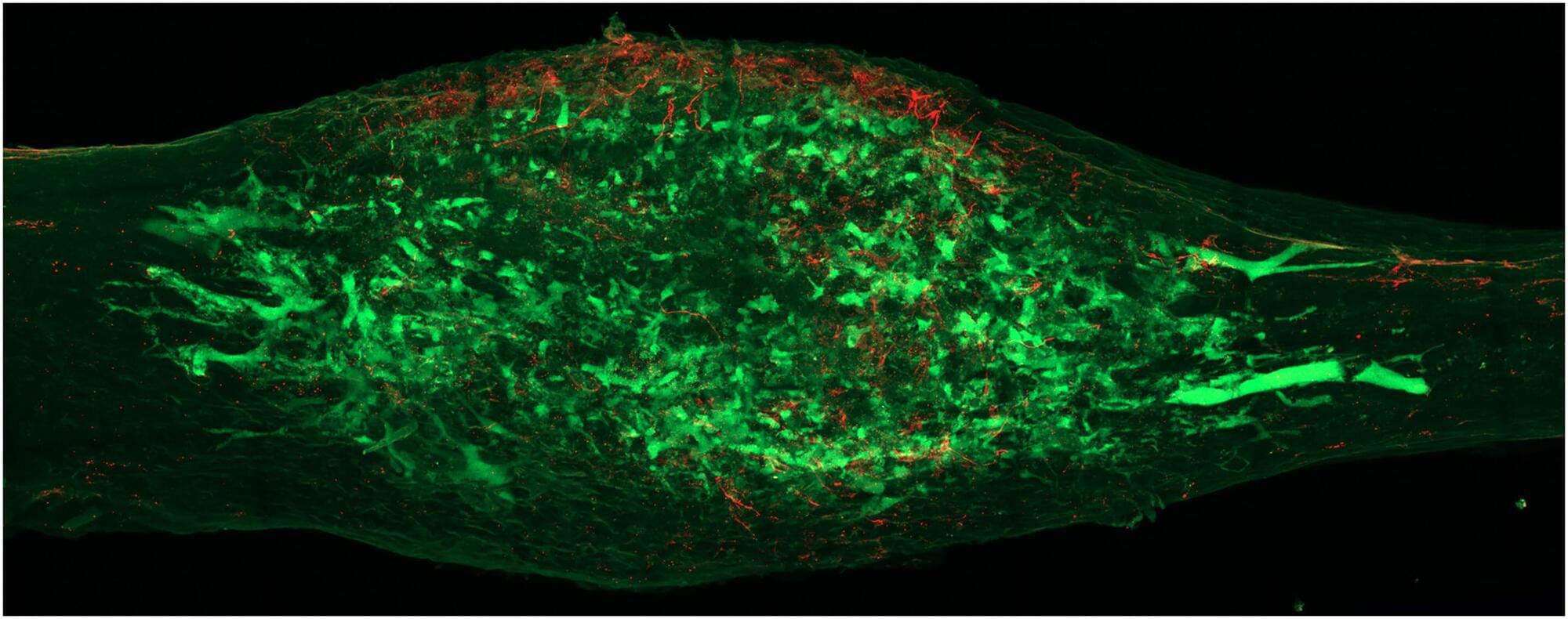
A humble Brazilian coastal plant used in folk medicine is now backed by science as a potential natural fighter against inflammation and arthritis.
A research team in Brazil has found strong evidence that the Joseph’s Coat plant (Alternanthera littoralis) is both safe and effective at reducing inflammation, easing pain, and protecting against arthritis. The study was carried out by scientists from the Federal University of Grande Dourados (UFGD), the State University of Campinas (UNICAMP), and São Paulo State University (UNESP).
Joseph’s Coat grows naturally along Brazil’s coastline and has long been used in traditional medicine to treat inflammation, infections, and parasitic illnesses. Despite its widespread use, there had been little scientific research confirming whether these benefits were real or whether the plant was safe.