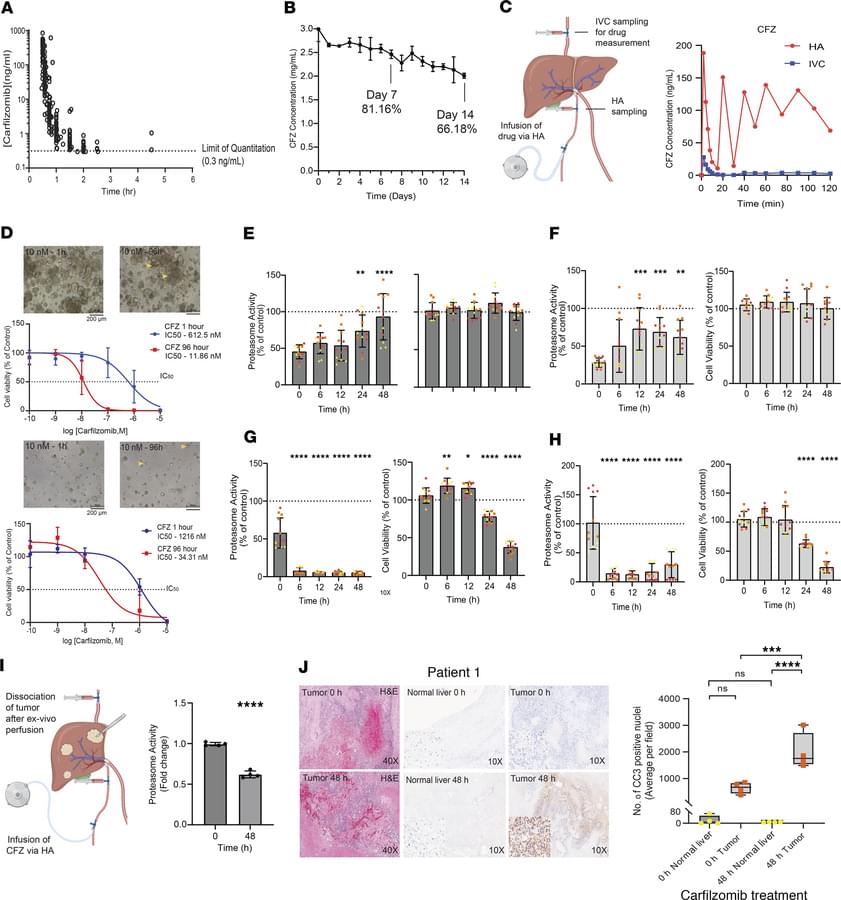
The first NMNH human trial shows NAD+ levels increased up to 3x in 90 days. Here’s what the data actually reveal—and what’s still missing.
Some links are affiliate links so we will earn a commission when they are used to purchase products.
If you would like to support our channel please consider joining our patreon / modernhealthspan.
~~~~~~~~~~~~~~
ProHealth (15% off: MODERN) https://prohealth.pxf.io/aObQRR NMNH 500mg.
Renue By Science (10% off: MHS) https://tinyurl.com/bdew4bfs NMN Powder • Lipo NR
BiOptimizers (15% off: MHBIO) https://bit.ly/47VAa8f — Magnesium Breakthrough.
Stemregen (15% off: MODERN) US only https://tinyurl.com/45z968yr.
Oxford Healthspan (15% off: MHS) https://tinyurl.com/hrxfnzpn — Spermidine.
Seeking Health (10% off: Richard10) https://crrnt.app/SEEK/-dm0MyrQ, Histamine Nutrients https://crrnt.app/SEEK/EpM7paAO
Qualia (15% off: MODERN) https://bit.ly/4jlaibe, Senolytic.
Wellness Extract (10% off: MODERNWE) http://wellnessextract.com/RICHARDWE Geranylgeraniol • Vit E
AX3 Life (20% off: MODERN20) https://tinyurl.com/2t3w26nw — Astaxanthin.
~~~~~~~~~~~~~~
The first human clinical trial results for NMNH are here. In this 90-day randomized, double-blind, placebo-controlled study, NMNH increased NAD+ levels up to 3x in healthy adults, with participants reporting improvements in energy and fatigue. But before you get too excited, there are important limitations to understand.
In this video, I break down the trial design, explain what the NAD+ increases actually mean, review the subjective outcomes like energy and emotional well-being (measured via SF-36), and discuss why the biological age claims are difficult to interpret. I also cover safety data and what we still need to know.
This is promising early data for NMNH vs NMN, but it’s unpublished, lacks key details, and needs independent replication. Here’s everything you need to know about what this trial does and doesn’t tell us.
Key topics: NMNH clinical trial, NAD+ boosters, NMN vs NMNH, longevity supplements, anti-aging research, NAD+ levels, UthPeak study, Phase I trial results.
📚 Chapters.
0:00 — Introduction & Trial Overview What this video covers and the trial basics.
1:39 — Trial Design & Methodology Study structure, participants, and objectives.
2:31 — NAD+ Results The primary outcome: dose-dependent increases.
3:18 — Subjective Outcomes & Limitations Energy, mood, biological age claims, and why interpretation is difficult.
6:26 — Safety & Final Thoughts Tolerability data and what comes next.
🌐Links in this video.
NMNH Clinical Trial https://www.clinicaltrials.gov/study/.… nicotinamide mononucleotide is a new and potent NAD+ precursor in mammalian cells and mice https://faseb.onlinelibrary.wiley.com… Reduced Nicotinamide Mononucleotide (NMNH) Potently Enhances NAD+ and Suppresses Glycolysis, the TCA Cycle, and Cell Growth https://pubmed.ncbi.nlm.nih.gov/33793… *************************************** Health claims Disclosure: Information provided on this video is not a substitute for direct, individual medical treatment or advice. Please consult with your doctor first. Products or services mentioned in this video are not a recommendation. Audio Copyright Disclaimer Please note that we have full authorization to the music that we used in our videos as they were created using the service WeVideo which provides the rights to the music. The rights are detailed in the terms of use that can be reviewed here https://www.wevideo.com/terms-of-use and any following inquiries should be addressed to [email protected]. ********************************************** #nmnh #nad #nmn.
Reduced nicotinamide mononucleotide is a new and potent NAD+ precursor in mammalian cells and mice.
https://faseb.onlinelibrary.wiley.com…
Reduced Nicotinamide Mononucleotide (NMNH) Potently Enhances NAD+ and Suppresses Glycolysis, the TCA Cycle, and Cell Growth.
https://pubmed.ncbi.nlm.nih.gov/33793…
*******************************