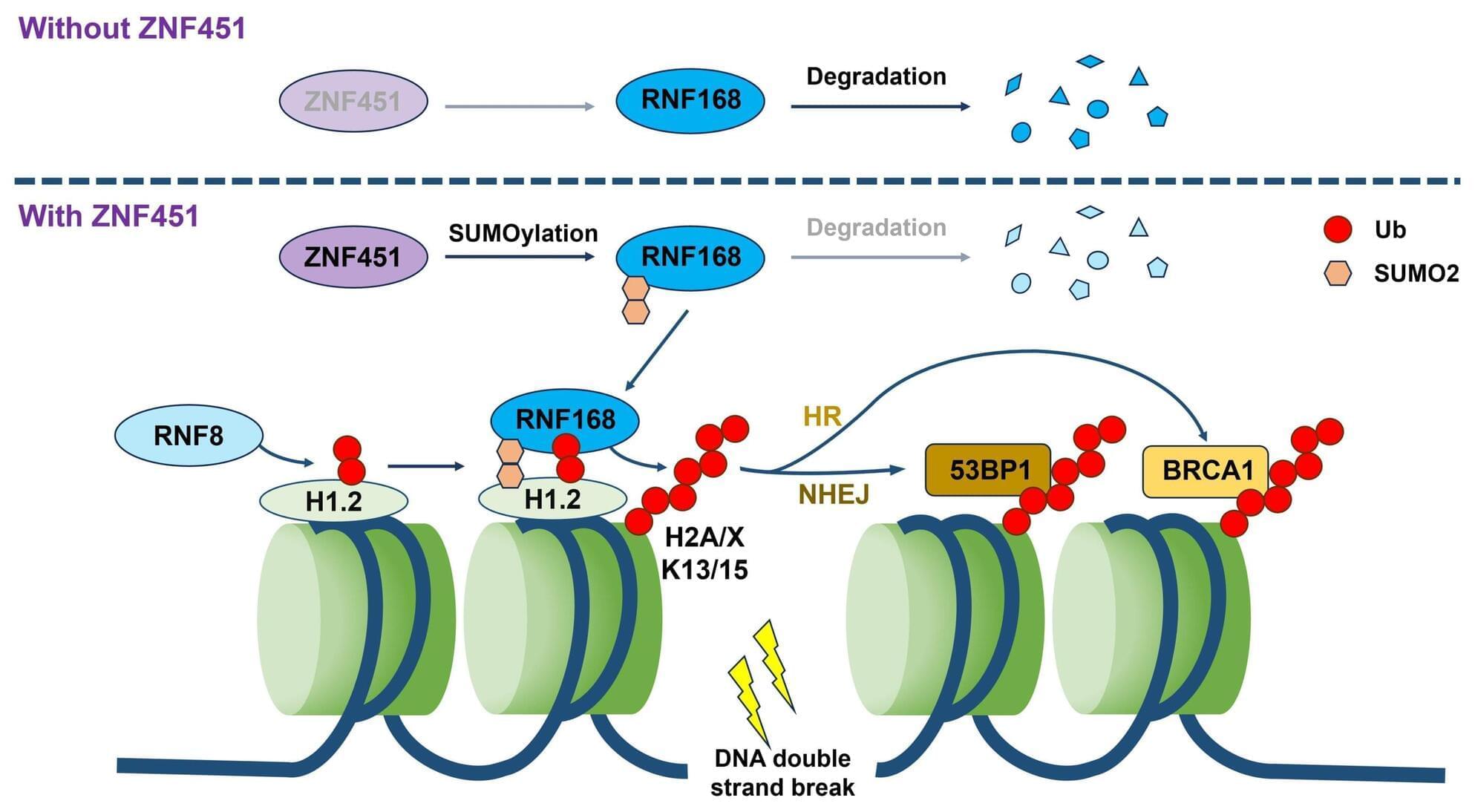
A team of infectious disease specialists and environmental engineers at Université Claude Bernard Lyon’s, École Centrale de Lyon, in France, and the University of Rome La Sapienza, in Italy, has found via experiments that the physical characteristics of exhaled droplets play a role in the transmission of infectious diseases.
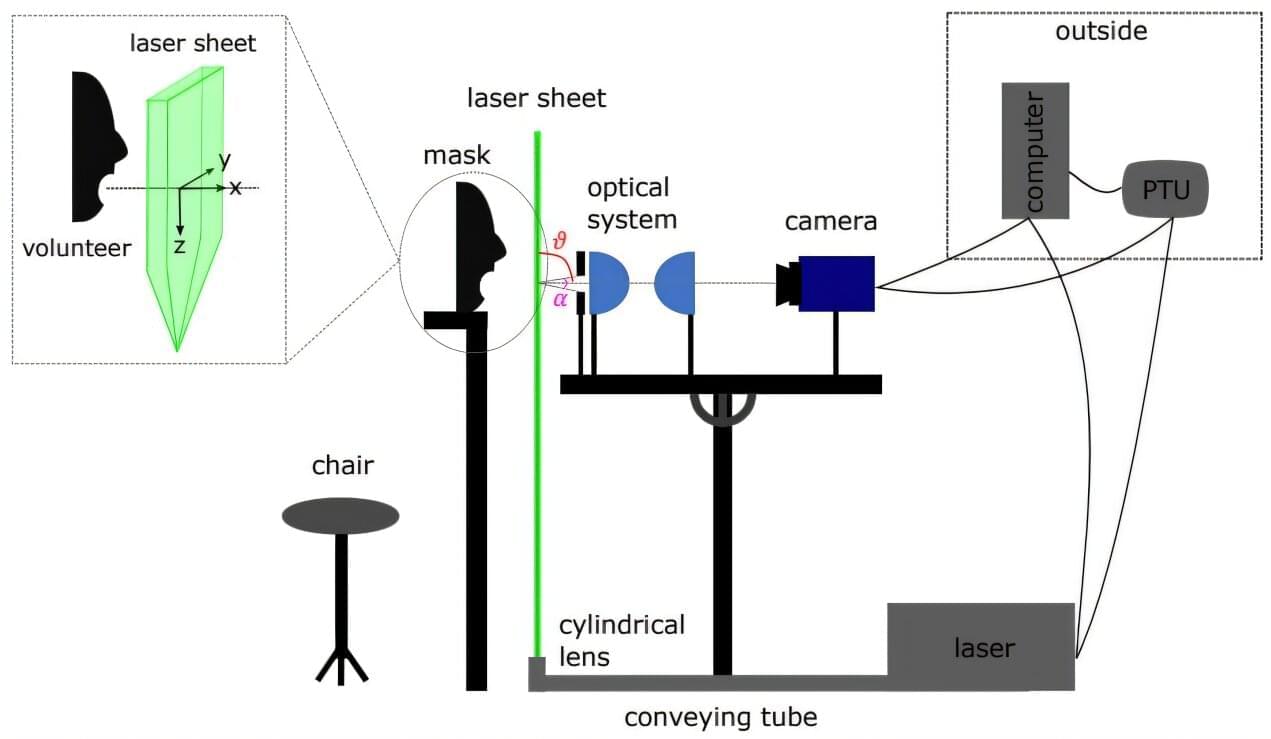
In their study published in the journal Physical Review Fluids, the group asked volunteers to breathe normally while silent, to speak normally, and to cough, while their exhausted droplet characteristics were measured.
Prior research has shown that many contagious diseases are spread via tiny droplets expelled through the nose and mouth of infected people as they breathe. These droplets are small enough to hang in the air long enough for others to inhale them, leading to infection. Prior research has also shown that some people can be characterized as superspreaders—for some reason, they infect more people than do others. Some suspect that this might be due to the size of droplets expelled by superspreaders or the distance the droplets travel once expelled.