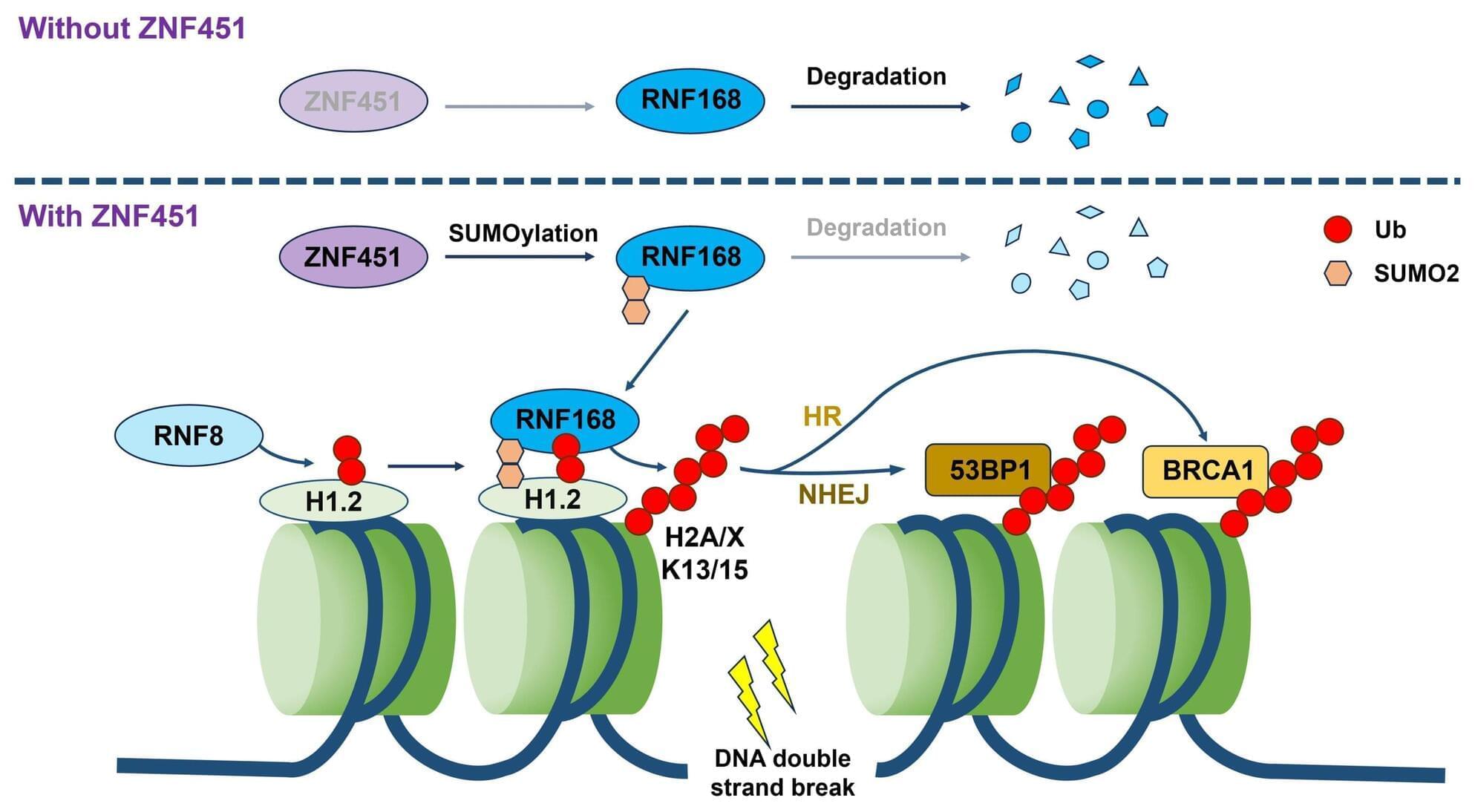
The pair decided to conduct a clinical trial that could be more compelling. In 12 people with early Alzheimer’s who took 3TC for 6 months, the drug didn’t boost cognitive abilities. But other indicators suggested some benefits, as Frost, Sullivan, and their colleagues revealed last month in npj Dementia. For instance, levels of one key neurodegeneration indicator dipped, suggesting 3TC protects patients’ brain cells. “That was the change I was most excited to see,” Frost says.
Their recent study was the first clinical test of an antitransposon strategy for Alzheimer’s to reach the finish line. But it’s just one of a growing number of trials launched by academic researchers and biotechs to gauge the effects of throttling transposons—so-called jumping genes. These vagrant sequences, some of which are relics of viruses that invaded cells long ago or may even be derived from symbiotic bacteria, make up more than 40% of the human genome but were once seen as largely harmless. However, a variety of evidence from human cell lines, lab animals, and epidemiological studies has implicated their antics in illnesses such as lupus, amyotrophic lateral sclerosis (ALS), Parkinson’s disease, and cancer, as well as in aging.
Encouraging results are trickling in. In 2022, a phase 2 trial determined that 3TC halted tumor growth in some patients with colorectal cancer. Last year, Transposon Therapeutics revealed that a different drug that stymies replication of these sequences slowed one sign of physical decline in people with ALS or another neurodegenerative disease, frontotemporal dementia. “It’s really amazing how quickly the story has developed,” says John Sedivy, a molecular biologist at Brown and the company’s co-founder.