A new study published in the journal Nutrients suggests that vitamin D levels play a “critical role” in preventing and treating colorectal cancer. The researchers share the key findings.




In this Perspective, the authors propose that patients with psoriatic arthritis and an inadequate response to therapy can be classified into two distinct subgroups, characterized by persistent inflammatory and non-inflammatory phenotypes, and discuss potential mechanisms underlying these phenotypes, as well as considerations for treatment strategies and trial design.
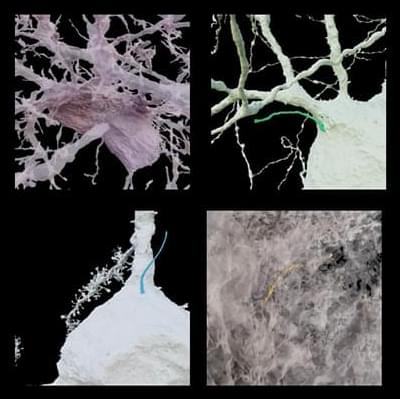
Many cells in our body have a single primary cilium, a micrometer-long, hair-like organelle protruding from the cell surface that transmits cellular signals. Cilia are important for regulating cellular processes, but because of their small size and number, it has been difficult for scientists to explore cilia in brain cells with traditional techniques, leaving their organization and function unclear.
In a new series of work, researchers at HHMI’s Janelia Research Campus, the Allen Institute, the University of Texas Southwestern Medical Center, and Harvard Medical School used super high-resolution 3D electron microscopy images of mouse brain tissue generated for creating connectomes to get the best look yet at primary cilia.
Non-personalized content and ads are influenced by things like the content you’re currently viewing and your location (ad serving is based on general location). Personalized content and ads can also include things like video recommendations, a customized YouTube homepage, and tailored ads based on past activity, like the videos you watch and the things you search for on YouTube. We also use cookies and data to tailor the experience to be age-appropriate, if relevant.
Select “More options” to see additional information, including details about managing your privacy settings. You can also visit g.co/privacytools at any time.

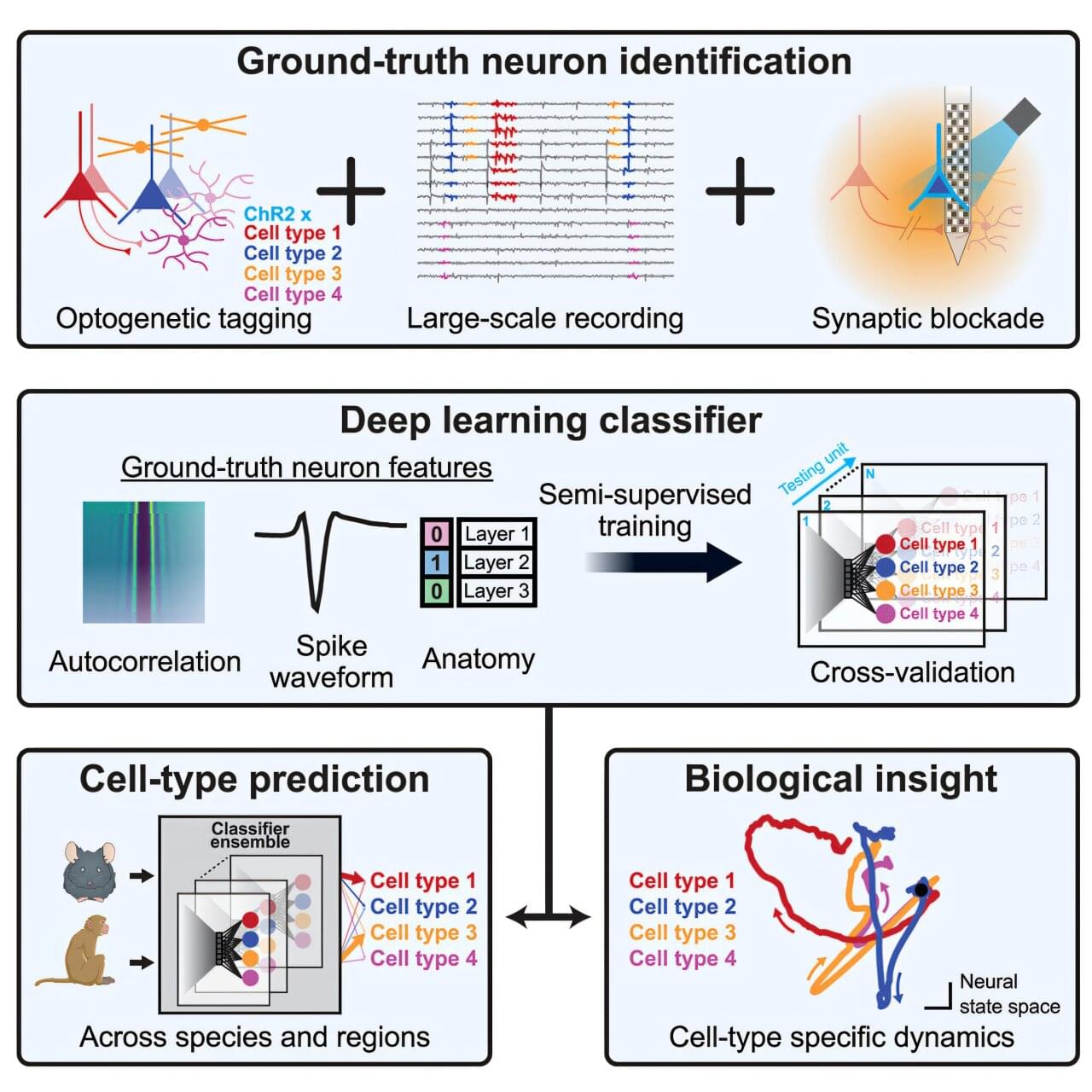
Understanding and treating brain disorders such as tremor, imbalance, and speech impairments requires deep knowledge of the cerebellum, a part of the brain that’s crucial for making accurate movements.
Scientists have long been able to eavesdrop on and record the electrical signals transmitted by neurons (brain cells) in the cerebellum, allowing them to observe the signals entering and exiting this region. But the computations that the brain performs between the input and output have been largely a mystery.
However, that is now changing. A team of researchers, including those from Baylor College of Medicine, have created an artificial intelligence tool that can identify the type of neuron producing electrical signals recorded from the cerebellum during behavior, allowing a new understanding of how the cerebellum works.

Fifty years since its discovery, scientists have finally worked out how a molecular machine found in mitochondria allows us to make the fuel we need from sugars, a process vital to all life on Earth.
Scientists at the Medical Research Council (MRC) Mitochondrial Biology Unit, University of Cambridge, have worked out the structure of this machine and shown how it operates like the lock on a canal to transport pyruvate—a molecule generated in the body from the breakdown of sugars—into our mitochondria.
Known as the mitochondrial pyruvate carrier, this molecular machine was first proposed to exist in 1971, but it has taken until now for scientists to visualize its structure at the atomic scale using cryo-electron microscopy, a technique used to magnify an image of an object to around 165,000 times its real size. Details are published in Science Advances.