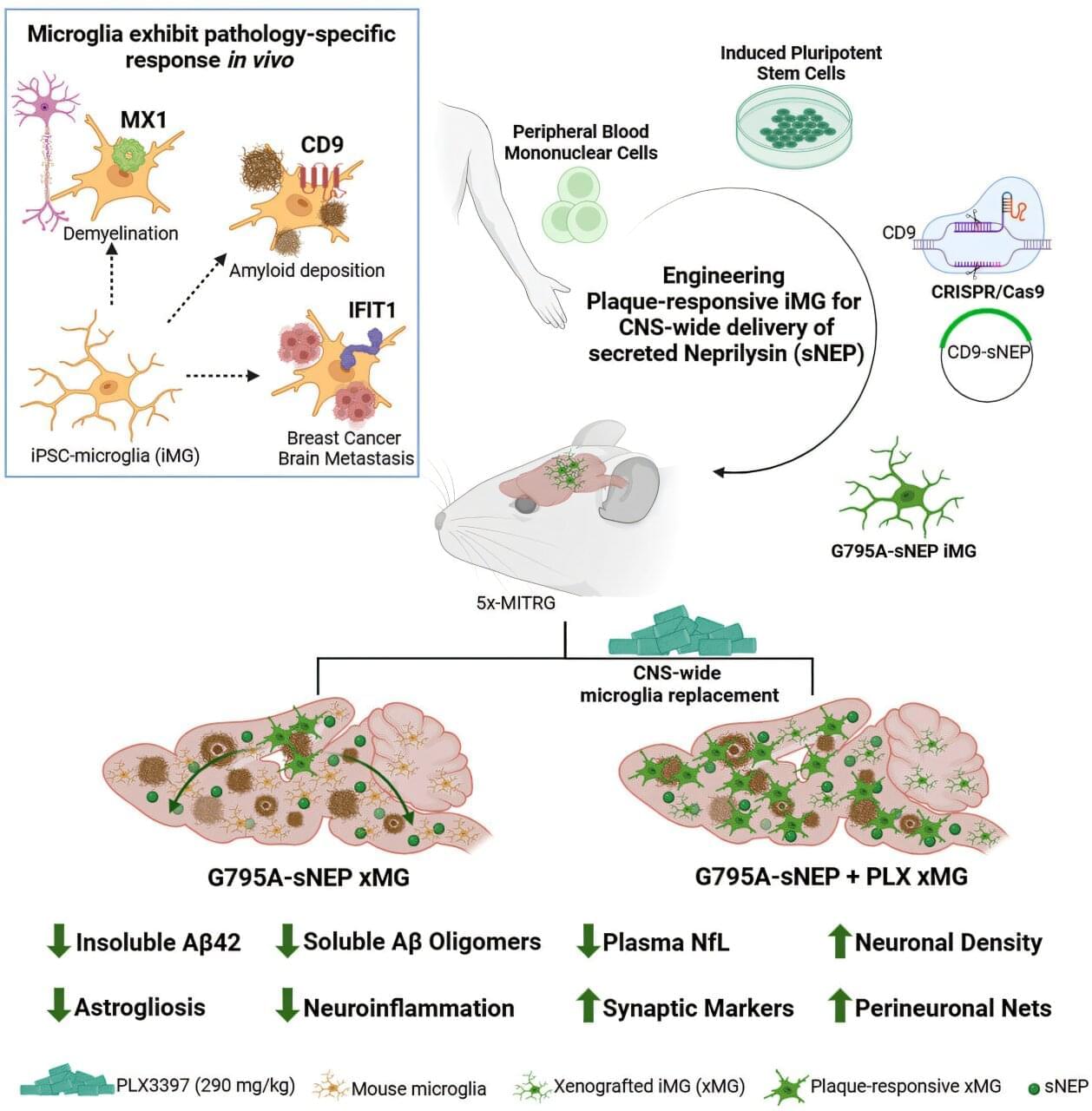
Vision is one of the most important human senses, yet more than 300 million people around the world are at risk of losing it due to various retinal diseases. Although recent treatments have helped slow the progression of these conditions, no effective therapy has been able to restore vision that has already been lost, until now. Researchers at KAIST have developed a new drug that successfully restores vision.
On March 30, KAIST announced that a research team headed by Professor Jin Woo Kim from the Department of Biological Sciences has created a treatment that regenerates retinal nerves to restore vision.