Category: biotech/medical – Page 427

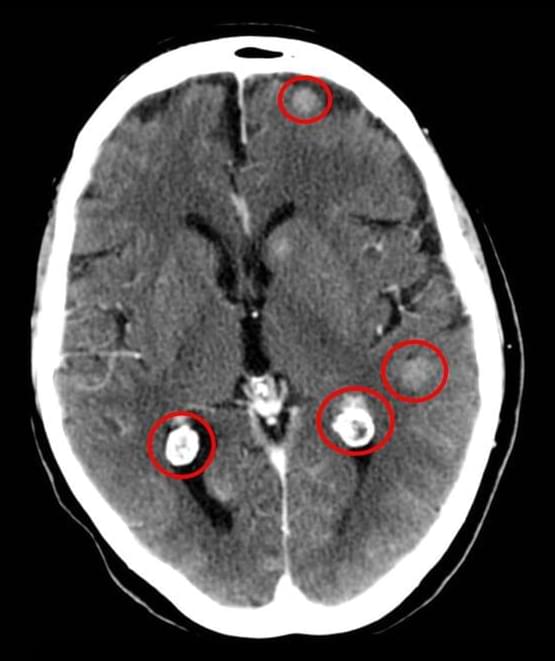
Lorbrena Effective as Initial Treatment of ALK-Positive NSCLC
The drug lorlatinib (Lorbrena) is superior to crizotinib (Xalkori) as an initial treatment for people with advanced non-small cell lung cancer (NSCLC) that has changes in the ALK gene, according to new results from a global clinical trial.
The findings are the latest from the CROWN study. Participants were randomly assigned to receive either lorlatinib or crizotinib as a treatment for advanced lung tumors with ALK gene mutations, a disease called ALK-positive lung cancer.
Several years ago, study investigators reported that participants who received lorlatinib went longer without the disease worsening, known as progression-free survival, than those who received crizotinib.
World’s first fully robotic double lung transplant performed at NYU
NYU Langone performs the world’s first fully robotic double lung transplant, marking a breakthrough in minimally invasive surgical care.
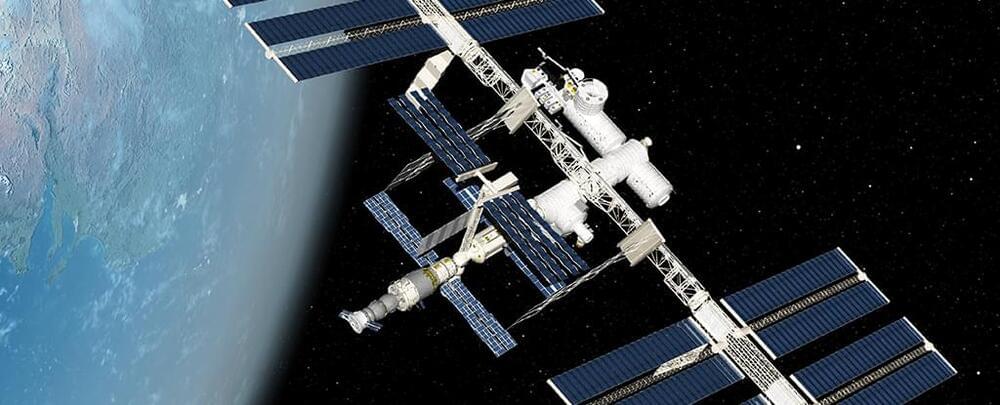
Stem Cells Grown in Space Turn Out to Have a Surprise Advantage
Stem cells are special in the way they can keep on replicating, and turn themselves into many other types of cell. Now scientists have discovered how their superpowers get a remarkable boost when they’re grown in space.
The microgravity environment increases some of the regenerative capacities of stem cells even further, researchers from the Mayo Clinic in Florida have found, based on experiments carried out on the International Space Station (ISS).
As stem cells play such a crucial role in the body’s repair process, with their ability to quickly replicate and differentiate, these findings could help in the study of disease prevention and treatment.
Lykke Sylow at ARDD2024: Preserving muscle mass for healthy aging: Old tricks and new targets
Lykke Sylow, University of Copenhagen, Denmark, presents at the 11th Aging Research and Drug Discovery meeting: Preserving muscle mass for healthy aging: Old tricks and new targets.

Improving cancer therapy using sonoenhancement with acoustic cluster therapy
Acoustic Cluster Therapy (ACT®)consists of clusters of gas-filled microbubbles and oil microdroplets.
During the last decade ACT® has been evaluated preclinically in various cancer models and combined with different drugs. It was first observed that ACT was able to increase the fluorescence from a tumor when sonoenhancement was combined with fluorescent macromolecules (Wamel 2016, Figure 1). Here it was found that already one minute after sonoenhancement, fluorescence had increased in the tumor compared to a non-treated control, followed by a fluorescence uptake that remained for several hours. Subsequently, ACT has been tested therapeutically in preclinical models of prostate cancer (Wamel 2016), pancreatic cancer (Kotopoulis 2016, Ng 2022), colon cancer (Bush 2019) and breast cancer (Bush 2020). The combination of ACT and drug was significantly better than the drug alone in all these studies, with quite large numbers of complete remissions. Combining ACT with the drug nab-paclitaxel for treatment of prostate cancer resulted in complete tumor remission in all tested animals. This shows that ACT provides sonoenhancement across very different cancer types and with different types of drugs, which increases the likelihood of seeing effects also in clinical trials as the tumor models collectively represents a variety of cancer biology.
Side effects and toxicity have also been tested in various small animal models during the last decade. During treatment, no bleeding or macroscopic damage was observed, and pathological evaluation has not identified microscopic damages. ACT was extensively tested for systemic toxicities, including studies in rats and dogs where ultrasound to the heart and liver was used to activate the ACT bubbles, and no significant adverse effects have been detected.
Sonoenhancement describes the actions of ACT. This is distinct from sonoporation describing the mode of action of ultrasound with the conventional free-flowing microbubbles, which are designed for ultrasound contrast enhancement. The mode of action of ACT is that the microclusters expand and lodge in tumor capillaries. This gives multiple effects in the tumor that can be separated into primary and secondary effects. The primary effect of ACT is the oscillations inside the capillaries, which affects the vascular wall and propagate into the extravascular domain of the tumor. This is clearly different from the action achieved with conventional microbubbles. An activated ACT bubble has a volume 1,000 times that of a conventional microbubble and a large contact area with endothelial cells. An activated ACT bubble will temporarily block the capillary and oscillations will affect the entire inner surface of the vessel. The volume of the bubble and the amplitude of the oscillations results in biomechanical work that is 1,000 times greater than that of conventional microbubbles.

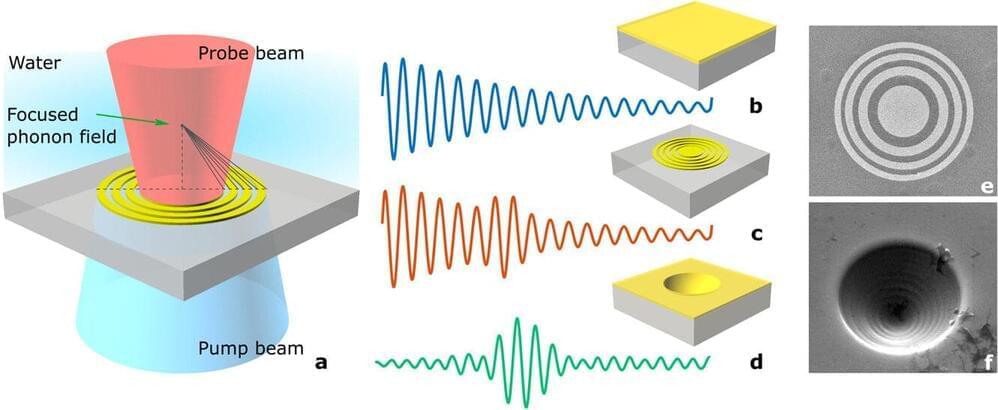
Improved ultrasound technique produces previously unattainable images inside live cells
A ultrasound technique from the University of Nottingham will allow the production of sharper images inside live cells without causing damage at resolutions that were previously unattainable.
The project, from the Faculty of Engineering’s Optics and Photonics research group, explores a way to look deep inside tiny structures, such as single cells, that regular light-based microscopes cannot, and without harming them. The work is published in the journal Photoacoustics.
This technique has been used to measure the stiffness of cancer cells at a single-cell level, which could allow for new methods of early cancer diagnosis to be developed.
Human Cell Atlas maps 100 million cells, advances medical research
The Human Cell Atlas aims to map the location, identity, and function of every cell in the human body by 2026.
Scientists with the Human Cell Atlas have profiled 100 million cells from over 10,000 people worldwide, aiming to map the human body down to the cellular level.