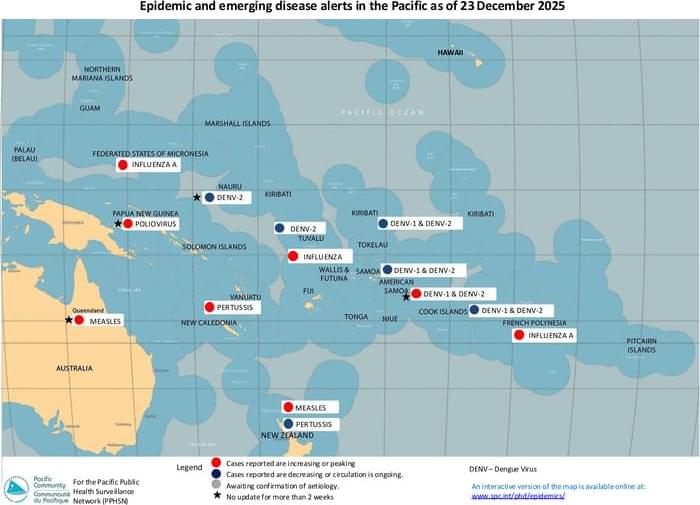
New York State Psychiatric Institute and Columbia University Medical Center investigators, with co-authors across additional US centers, report greater cognitive worsening at 78 weeks with valacyclovir than with placebo among adults with early symptomatic Alzheimer’s disease and herpes simplex virus (HSV) seropositivity.
Infectious diseases may contribute to Alzheimer’s disease pathogenesis. HSV-1 can become latent after oral infection, enter the brain via retrograde axonal transport, infiltrate the locus coeruleus, and migrate to the temporal lobe.
β-amyloid plaques and tau neurofibrillary tangles are neuropathological features of Alzheimer’s disease. Animal models connect HSV-1 infection of neuronal and glial cells with a decrease in amyloid precursor protein, an increase in intracellular amyloid β-protein, and phosphorylation of tau protein.