Inflammation covertly rewires the bone marrow, enabling mutated stem cells to rise and setting the stage for future blood disease.


Think of cells as the biological answer to battery-powered electronics. Mitochondria are the batteries that supply them with enough energy to keep going. Unfortunately, just like the two standard AAs in your remote control, they eventually run out of power and die—but (much like actual batteries) they can also be recharged and replaced.
Breakdown of mitochondria causes cells to glitch. Wear and tear can happen with age, usually from years of exposure to free radicals that cause oxidative stress and inflammation, but can also be caused by injury from degenerative diseases or mitochondrial toxicity from certain drugs and other harmful substances. When there is damage to the cell, mitochondria begin to lose their capacity to generate energy. Losing mitochondria is detrimental to cell function. This is why biomedical engineer Akhilesh Gaharwar and his research team at Texas A&M University have come up with a way to regenerate them.
A research team at Oregon Health & Science University has discovered a promising new drug combination that may help people with acute myeloid leukemia overcome resistance to one of the most common frontline therapies.
In a study published in Cell Reports Medicine, researchers analyzed more than 300 acute myeloid leukemia, or AML, patient samples and found that pairing venetoclax, a standard AML drug, with palbociclib, a cell-cycle inhibitor currently approved for breast cancer, produced significantly stronger and more durable anti-leukemia activity than venetoclax alone. The findings were confirmed in human tissue samples, as well as in mouse models carrying human leukemia cells.
“Of the 25 drug combinations tested, venetoclax plus palbociclib was the most effective. That really motivated us to dig deeper into why it works so well—and why it appears to overcome resistance seen with current therapy,” said Melissa Stewart, Ph.D., research assistant professor at OHSU and lead author of the study.
A robot revolution, driven by advancements in robotics and AI, is imminent and will drastically transform the economy, labor, and society, leading to a post-labor, post-scarcity system with abundant energy and labor ##
## Questions to inspire discussion.
Investment & National Strategy.
🚀 Q: Why should governments prioritize humanoid robot investment now? A: Governments must treat humanoid robots as a national priority for transforming productivity and defense, with enormous investments justified because there’s no time to lose as both the US and China have already recognized this imperative.
💰 Q: What economic growth rates become possible with early humanoid robot adoption? A: Spinning up the humanoid robot flywheel early enables exponential economic growth rates of 20–100% per year, unlocking unprecedented prosperity and catapulting societies up the curve over the next 15 years.
⚡ Q: Which countries or entities will likely lead the humanoid robot transformation? A: Outsiders rather than incumbents or centers of power will lead the transformation to a new economic paradigm, as history shows leadership typically comes from the edge rather than the status quo.
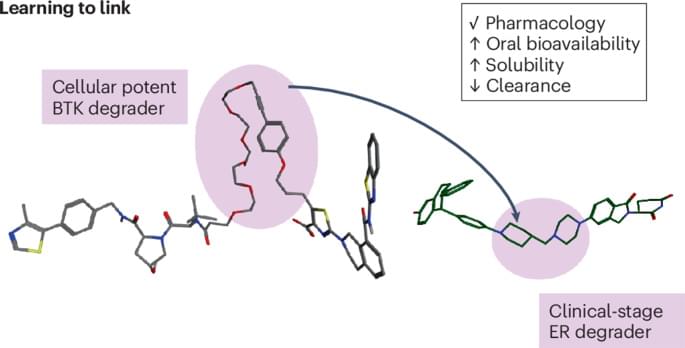
Proteolysis targeting chimeras (PROTACs) are an emerging platform in drug discovery with the potential to unlock novel pharmacology and tackle undruggable targets. This Review highlights learnings from the first cohort of clinical-stage PROTACs, which use short, ring-rich linkers, often complemented with one basic centre, to achieve good bioavailability and metabolic stability.
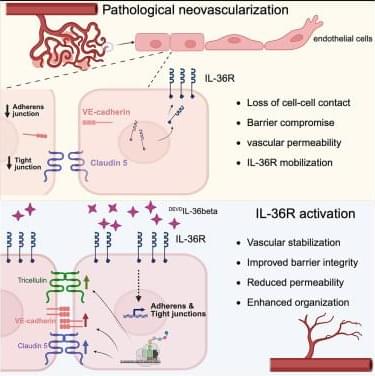
Only a small number of barrier-enhancing factors have been discovered to specifically increase barrier integrity, enhance vessel stability, and make vessels resistant to fluid leakage. Here, Fahey et al. present DEVDIL-36β as a cytokine promoter of vascular integrity in the CNS, with barrier-enhancing properties through upregulation of junctional components.



The device is also used in selected neurological cases where accurate signal detection and responsive stimulation are critical to managing symptoms over time. Its application forms part of a broader treatment pathway rather than a standalone intervention.
Implantation is performed using minimally invasive techniques and typically takes three to five hours. The approach avoids large surgical incisions and supports shorter recovery periods, allowing patients to resume daily activities more quickly.
Rather than marking a single milestone, the continued use of this technology reflects KFSHRC’s integration of artificial intelligence into routine neurological care, where adaptability and long-term management are central to patient outcomes.
RIYADH, SAUDI ARABIA, December 28, 2025 /EINPresswire.com/ — At King Faisal Specialist Hospital & Research Centre (KFSHRC) in Riyadh, artificial intelligence enabled brain implants are used as part of advanced care for patients with neurological conditions, including Parkinson’s disease and selected movement disorders.
The implant functions by continuously analyzing brain signals and responding to abnormal activity through targeted electrical stimulation. This adaptive approach allows treatment to adjust in real time based on the patient’s neural patterns, reducing reliance on fixed stimulation settings and limiting the need for frequent manual recalibration.
In clinical practice, the technology has supported improved symptom control for patients whose conditions require precise neuromodulation. As treatment progresses, some patients have been able to reduce their dependence on medication under clinical supervision, while maintaining daily function and stability.
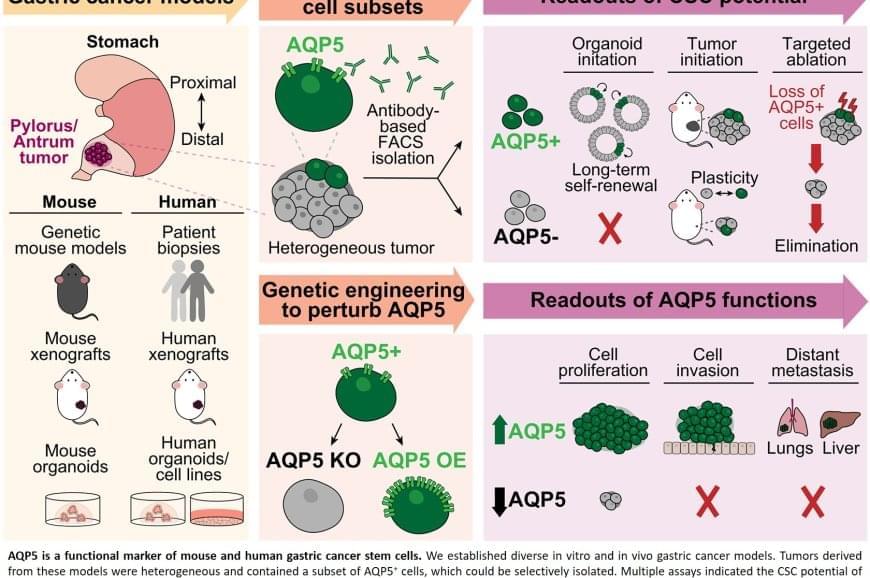
Scientists have long suspected that a small population of cells survives treatment and regenerates the tumor. These “cancer stem cells” are thought to resist conventional therapies, allowing the disease to return even after the visible tumor has been removed.
Previous attempts to identify gastric cancer stem cells using other protein markers, such as CD44 or CD133, yielded inconsistent results. These markers often appeared on healthy cells as well or did not fully account for tumor behaviour.
The team discovered that AQP5 reliably marks the cancer stem cells in gastric tumors. Aquaporins are proteins that form channels in cell membranes to control the movement of water into and out of cells. While AQP5 was previously known to mark stem cells in healthy gastric tissue, this study shows it also identifies the specific cells responsible for driving tumor growth, spread, and recurrence.
Importantly, AQP5 does more than simply mark these cells; it actively contributes to their aggressive behavior.
The researchers found that cells with AQP5 were capable of forming new tumors, while cells without AQP5 rarely did so. Most significantly, when they used a targeted method to eliminate only the AQP5-expressing cells, tumors stopped growing or shrank entirely and did not recur. This held true even for cancers that had spread to other organs.
Scientists have identified the specific cells responsible for gastric cancer’s tendency to return after treatment. The study also demonstrated that eliminating these cells stops tumors from growing, even in advanced disease that has spread to other organs.