If a patient doesn’t respond well to GLP-1s, there are other things to try. But ultimately, the medications just might not work for them.


Researchers have developed a new computational approach that uncovers possible drugs for specific cellular targets for treating glioblastoma, a lethal brain tumor. This approach enabled them to predict more effective treatment combinations to fight the disease on an individualized basis.
This laboratory and computational research effort was led by scientists at Georgetown’s Lombardi Comprehensive Cancer Center.
“The cellular targets we identified could be key to effectively fighting a disease that has seen only one new targeted drug approved in the last two decades,” says Nagi G. Ayad, Ph.D., senior author, associate director for translational research, and professor of oncology at Georgetown Lombardi.
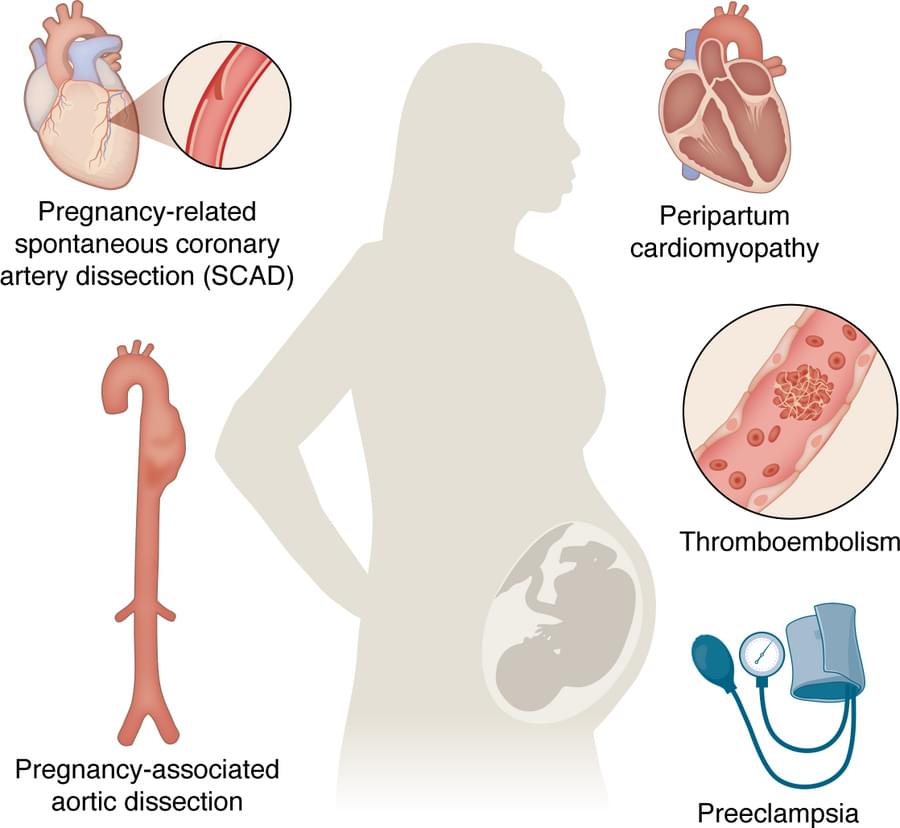
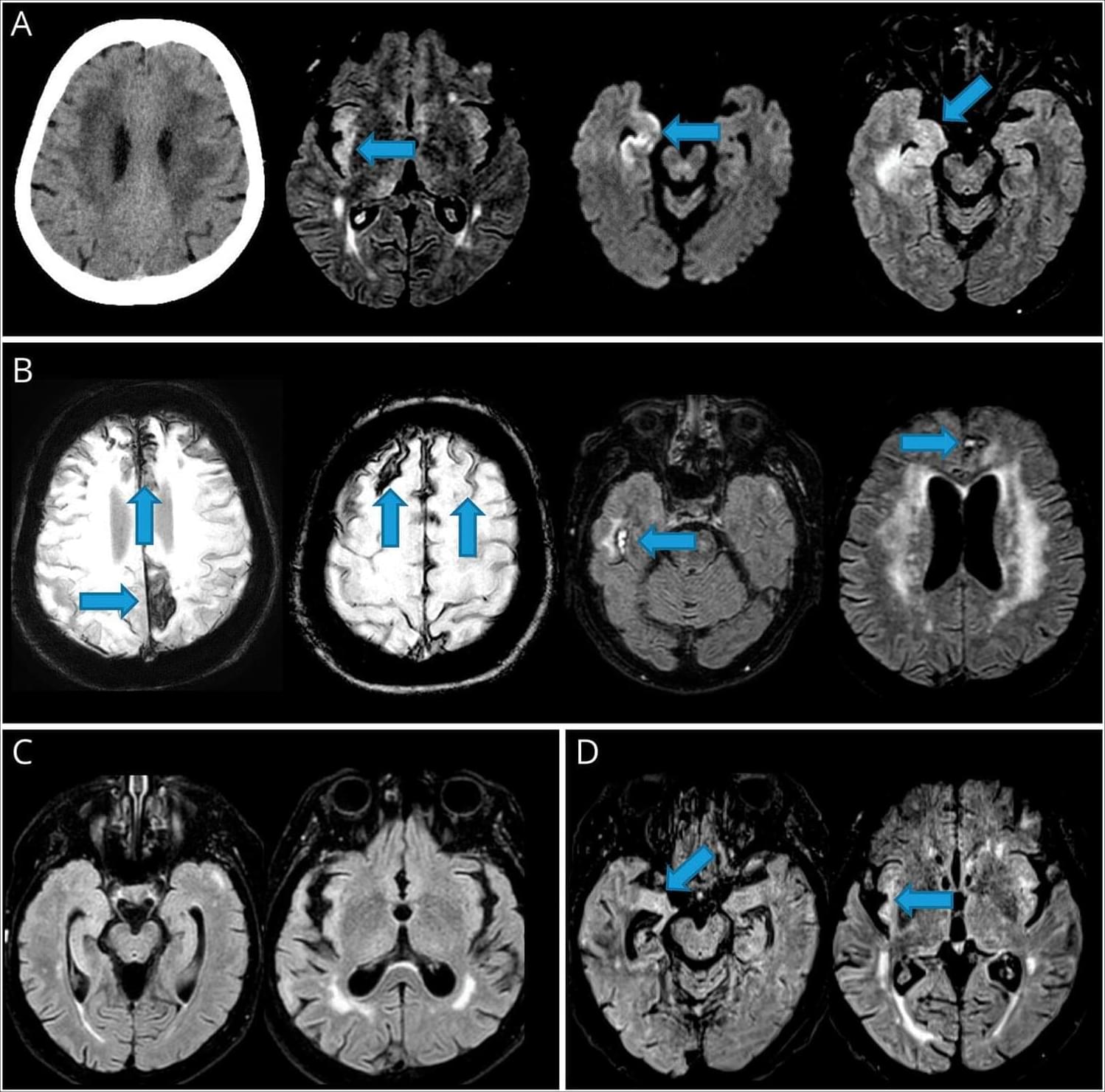
Yijun Yang, Jennifer Lewey & Zoltan Arany explore pregnancy-related cardiovascular adaptations and complications, along with the mechanisms, animal models, and racial and ethnic disparities tied to these conditions.
1Cardiovascular Institute, and.
2Division of Cardiology, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Address correspondence to: Zoltan Arany, 11th floor Smilow Translational Research Center, 34th and Civic Center Blvd, Philadelphia, Pennsylvania 19,014, USA. Email: [email protected].
Cells have a remarkable housekeeping system: Proteins that are no longer needed, defective, or potentially harmful are labeled with a molecular “tag” and dismantled in the cellular recycling machinery. This process, known as the ubiquitin-proteasome system, is crucial for health and survival.
Now, an international team of scientists led by CeMM, AITHYRA and the Max Planck Institute of Molecular Physiology in Dortmund has identified a new class of small molecules that harness this natural system to accelerate the removal of an immune-modulating enzyme called IDO1.
The findings, published in Nature Chemistry, introduce a new concept in drug discovery that could transform how we target difficult proteins in cancer and beyond.

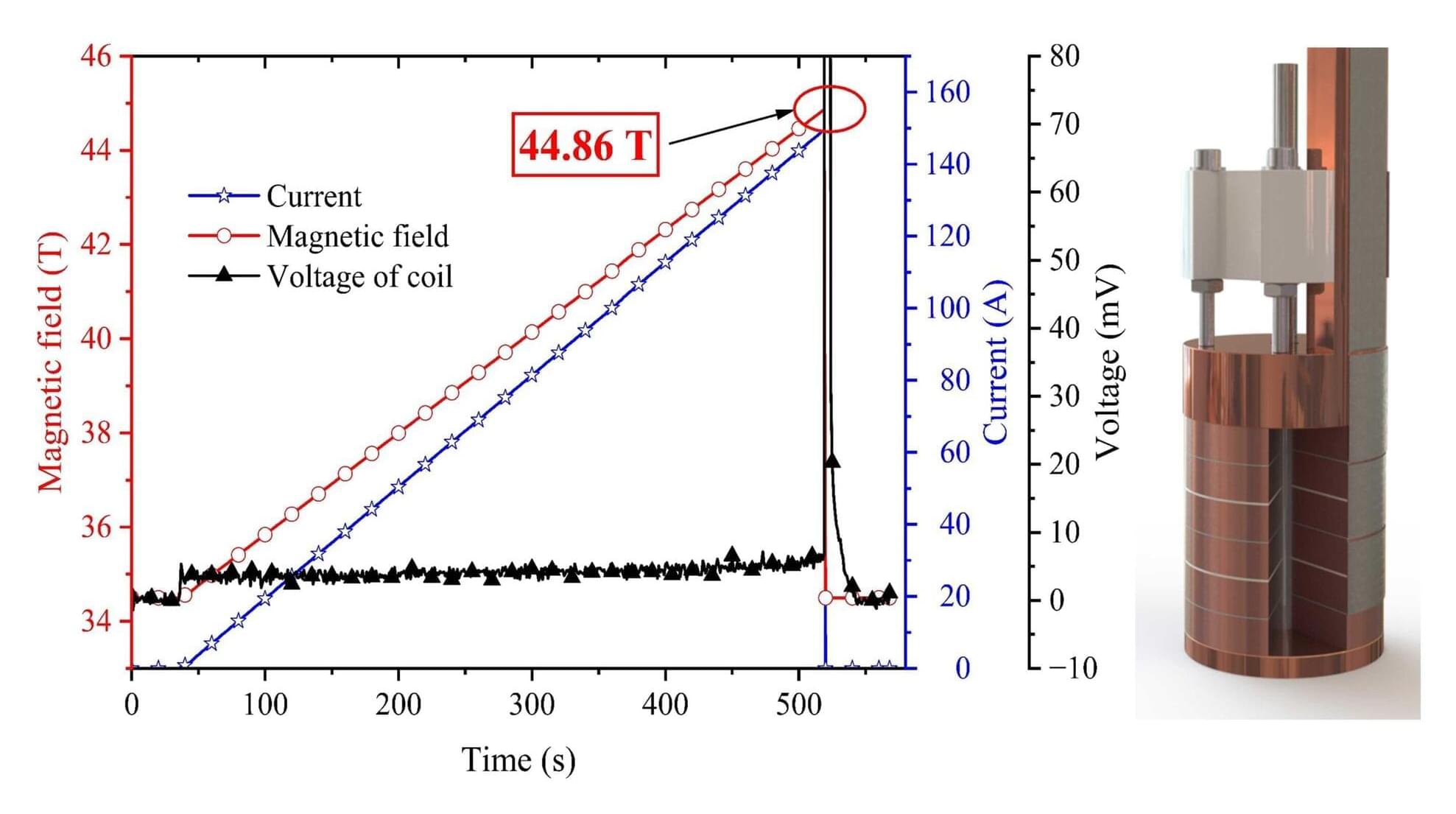
A research team led by Kuang Guangli and Jiang Donghui at the High Magnetic Field Laboratory of the Hefei Institutes of Physical Science of the Chinese Academy of Sciences (CHMFL), has developed a “pocket-type” high-temperature superconducting (HTS) coil, achieving a record combined magnetic field of 44.86 tesla.
The coil, wound using domestically produced REBa₂Cu₃O₇₋ₓ (REBCO) tapes, generated 28.20 T at zero field in a liquid helium bath and produced an additional 10.36 T inside the 34.5 T steady-state magnetic field of the WM5 water-cooled magnet.
Steady high magnetic fields are critical for frontier research in materials science, physics, and biology, enabling scientists to observe new phenomena and explore new laws of matter. REBCO high-temperature superconducting material has become one of the optimal choices for developing devices that generate higher magnetic fields, owing to its high current-carrying capacity and favorable mechanical properties.
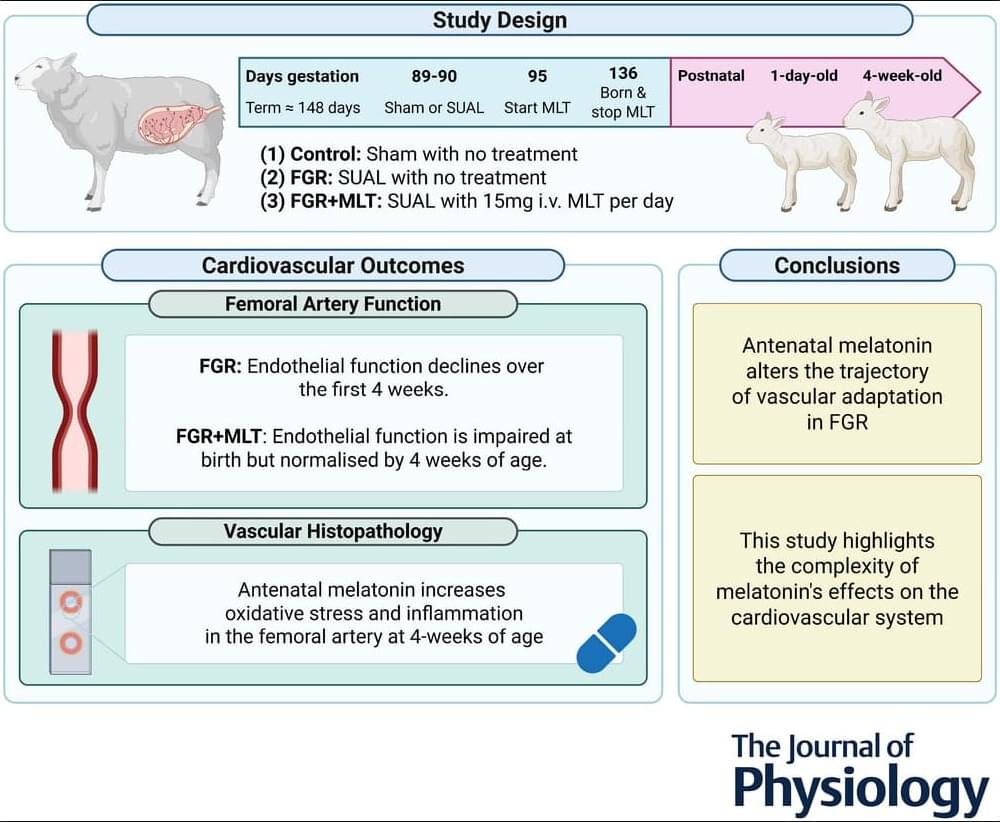
Recent Research by Charmaine R. Rock of Hudson Institute of Medical Research et al. examines antenatal melatonin for cardiovascular deficits in fetal growth restriction 🫀 💊
🔗 📜 Read the study here.
The results of the present study indicate that melatonin has the potential to mitigate the progressive development of impaired endothelium-dependent vasodilatation in growth-restricted lambs. However, this benefit is associated with transient impairment of endothelial function on the first day of life. It would be prudent to investigate the physiological implications of this early vascular dysfunction during the critical period of cardiovascular adaptation to postnatal life. Adjustments to the antenatal melatonin treatment regime, such as tapering the dose before and after birth, could be explored to support a smoother cardiovascular transition on the first day of life.
Additionally, because the present study was conducted up to 4 weeks of age, equivalent to an ∼1-year-old human infant, extending the study outcomes into adulthood is important to determine whether endothelial function is sustained or continues to improve with age, potentially achieving full restoration to the relaxation abilities observed in control lambs. Furthermore, given that functional assessment of the carotid artery was not possible in our study, future research should include such testing to provide a more comprehensive understanding of how FGR impacts vascular function.
The present study provides novel insight into the short-and longer-term, and region-specific impact of melatonin on the cardiovascular system. Our findings demonstrate that, although antenatal melatonin improves the contribution of NO to endothelium-dependent vasodilatation in the femoral artery of newborn FGR+MLT lambs, it is accompanied by an overall reduction in femoral endothelium-dependent vasodilatory capacity. Notably, this reduction in endothelial function is transient and improves by 4 weeks of age, which contrasts with the progressive impairment of endothelial function seen in untreated FGR lambs. Immunohistochemical analysis revealed elevated oxidative stress and inflammatory markers in the femoral artery of 4-week-old FGR+MLT lambs.
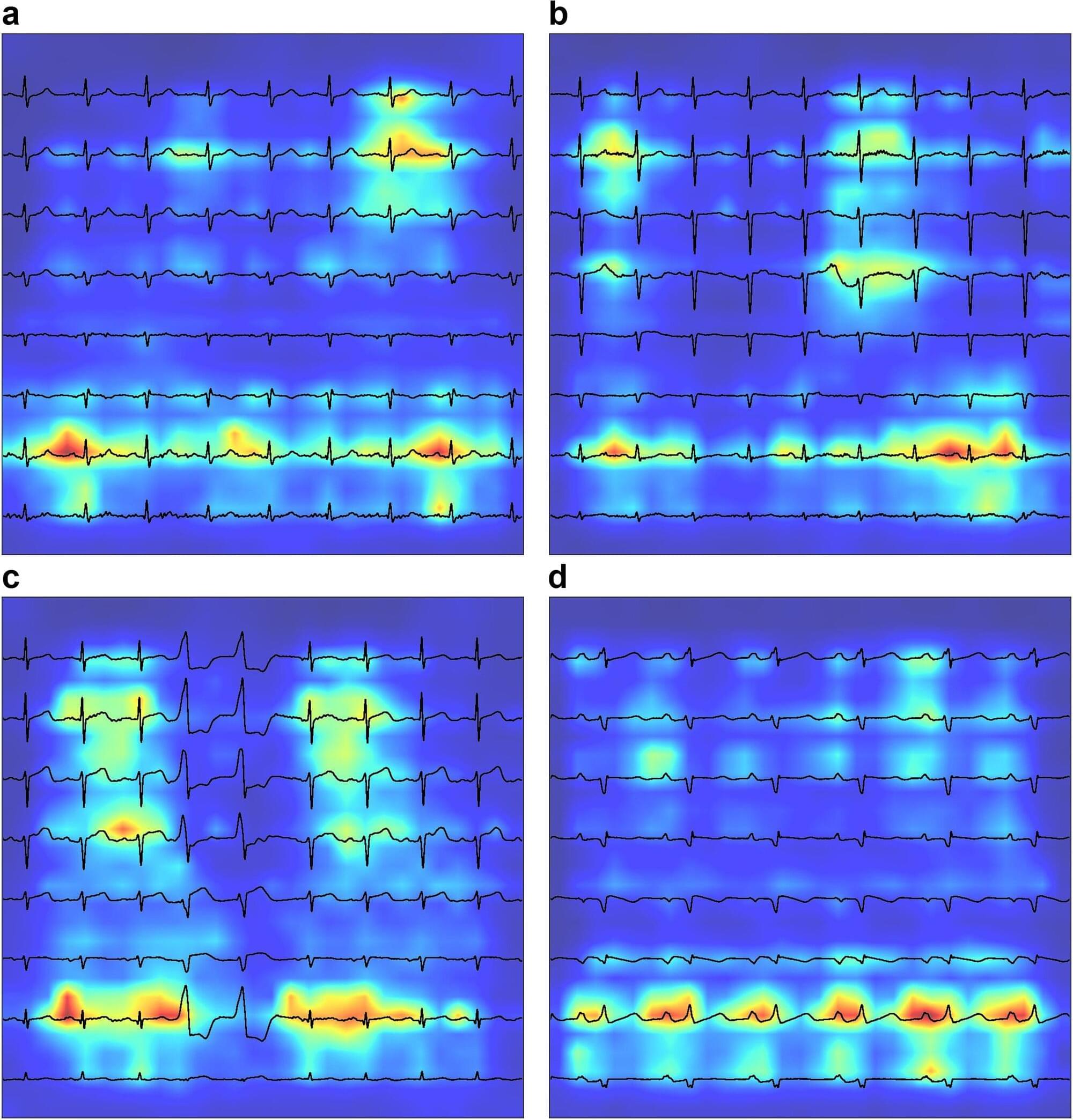
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality globally. Effective management hinges on early diagnosis, which is often impeded by non-specific symptoms and resource-intensive diagnostic methods. A study published in the journal eBioMedicine assesses the effectiveness of electrocardiograms (ECGs) analyzed via deep learning as a tool for early COPD detection.
Mount Sinai researchers utilized a Convolutional Neural Network model to analyze ECGs, or medical tests that record the heart’s electrical activity, and can detect COPD. The primary outcome was the accuracy of a new clinical COPD diagnosis, as determined by International Classification of Disease codes. They performed an evaluation using Area-Under-the-Curve (AUC) metrics, derived by testing against ECGs from patients at five hospitals within the Mount Sinai Health System who represented a demographically diverse patient population in New York City.
They examined data from 2006 to 2023 within the GE MUSE system that exports electrocardiograms as individual XML files containing raw waveforms. The experts also used ECGs from patients at another hospital and ECGs of patients with COPD within the UK BioBank to expand the cohort and validate the analysis.