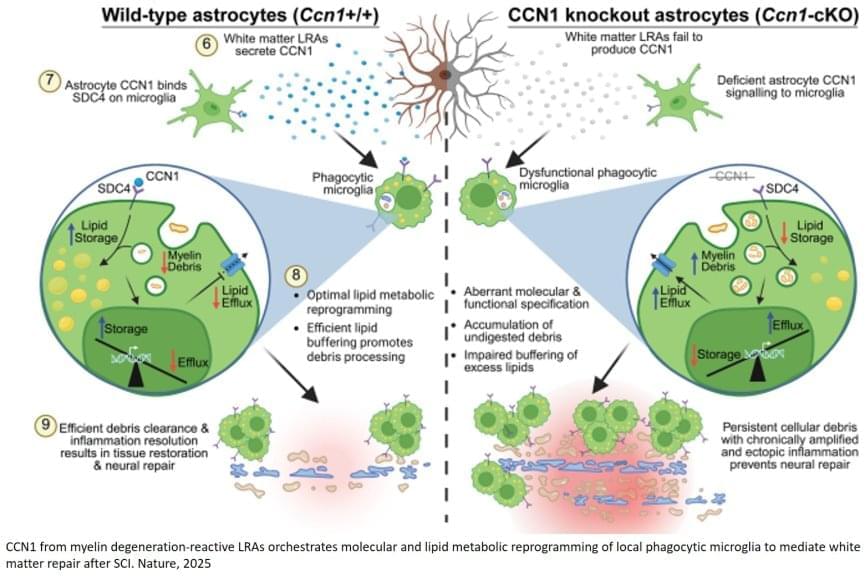
Investigators looked at laboratory mice with spinal cord injury and found that lesion-remote astrocytes (LRAs) play an important role in supporting nervous system repair. They saw strong evidence of the same mechanism in tissue samples from human patients with spinal cord injury.
The Lab identified one LRA subtype that sends out a protein called CCN1 to signal to immune cells called microglia.
“One function of microglia is to serve as chief garbage collectors in the central nervous system,” the senior author said. “After tissue damage, they eat up pieces of nerve fiber debris—which are very fatty and can cause them to get a kind of indigestion. Our experiments showed that astrocyte CCN1 signals the microglia to change their metabolism so they can better digest all that fat.”
The author said this efficient debris clearing might have a role in the spontaneous recovery found in many patients with spinal cord injury. In the absence of the astrocyte-derived CCN1 protein, the investigators found that recovery is drastically impaired.
“If we remove astrocyte CCN1, the microglia eat, but they don’t digest. They call in more microglia, which also eat but don’t digest,” the author said. “Big clusters of debris-filled microglia form, heightening inflammation up and down the spinal cord. And when that happens, the tissue doesn’t repair as well.”
When investigators looked at spinal cord tissue from human patients with multiple sclerosis, they found the same mechanism at work, the author said and added that these fundamental principles of tissue repair likely apply to any sort of injury of the brain or spinal cord.