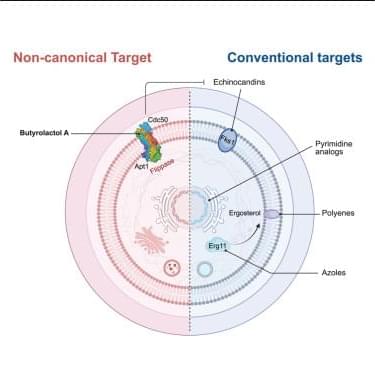
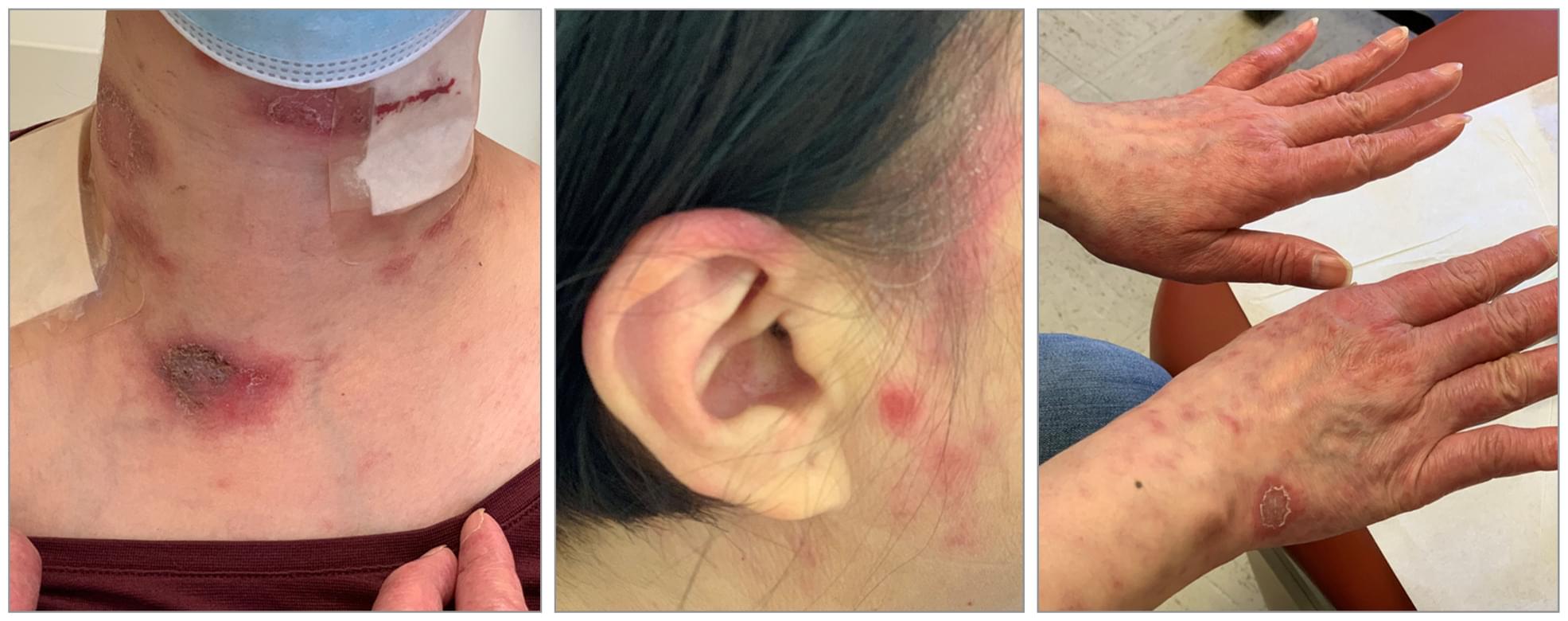
Immune checkpoint inhibitors can cause a wide spectrum of immune-related adverse events, including rare but severe neurological complications. This report describes a fatal case of progressive immune-mediated neuropathy in a melanoma patient treated with PD-1 blockade, in whom worsening neuropathic pain and functional decline coincided with irregular, non-aligned timing of immunotherapy infusions. PET-CT imaging, MRI, and laboratory testing excluded metastatic, infectious, metabolic, and paraneoplastic causes. Longitudinal evaluation revealed fluctuations in inflammatory indices, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII), with peaks occurring shortly after late-afternoon infusions. Earlier infusions were associated with fewer symptoms.