We all want to know how to live longer, but is a prolonged life a healthy, happy one? One Vogue writer looks at the science that says it might be possible.


The first 200 people to sign up with Brilliant using my link will get 20% off the annual subscription!
http://brilliant.org/thethoughtemporium
_________________________________________________________________________
What started as a dream more than 10 years ago, has finally become reality. After more than 2 years of work, dozen of failures, hundreds of hours of lab work and design time, we’ve finally done it. We’ve engineered a strain of yeast that produce real spider silk! This video explains how.
Check out our new merch: https://teespring.com/stores/the-thought-emporium
Previous videos:
Project overview — https://www.youtube.com/watch?v=Fx8TcGrCOSI
DNA extraction — https://www.youtube.com/watch?v=knosqmvLWSc
PCR — https://www.youtube.com/watch?v=7kJ2o7P8D00
Journey to the Microcosmos — https://www.youtube.com/channel/UCBbnbBWJtwsf0jLGUwX5Q3g
_________________________________________________________________________
Support the show and future projects:
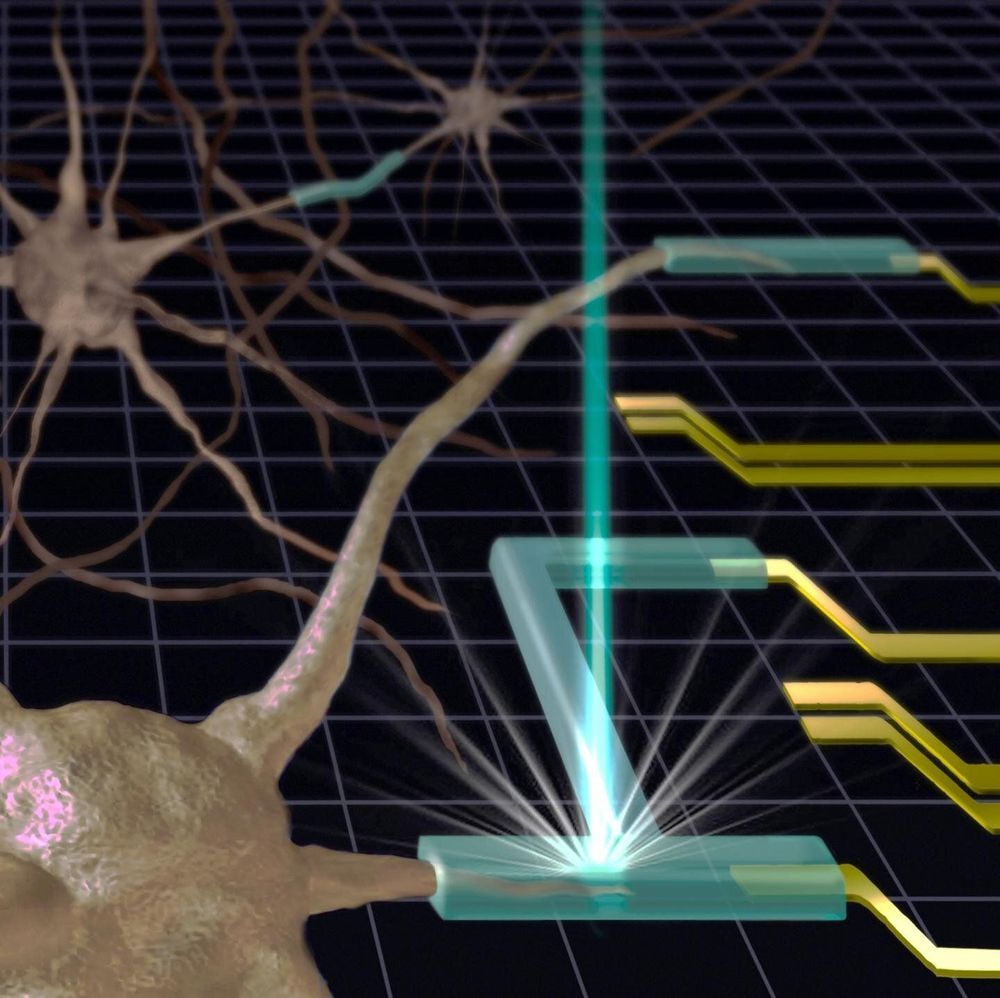
Researchers at the National Institute of Standards and Technology (NIST) have developed a new method of 3D-printing gels and other soft materials. Published in a new paper, it has the potential to create complex structures with nanometer-scale precision. Because many gels are compatible with living cells, the new method could jump-start the production of soft tiny medical devices such as drug delivery systems or flexible electrodes that can be inserted into the human body.
A standard 3D printer makes solid structures by creating sheets of material—typically plastic or rubber—and building them up layer by layer, like a lasagna, until the entire object is created.
Using a 3D printer to fabricate an object made of gel is a “bit more of a delicate cooking process,” said NIST researcher Andrei Kolmakov. In the standard method, the 3D printer chamber is filled with a soup of long-chain polymers—long groups of molecules bonded together—dissolved in water. Then “spices” are added—special molecules that are sensitive to light. When light from the 3D printer activates those special molecules, they stitch together the chains of polymers so that they form a fluffy weblike structure. This scaffolding, still surrounded by liquid water, is the gel.
Short excerpt of a recent interview with Dr. David Sinclair published in the Youtube channel “Think Inc.”
Short excerpt of an interview with Dr. David Sinclair published in the Youtube channel “Think Inc.“
During the interview, Dr. Sinclair referres to the possibility of turning back the biological aging clock of the entire human body, through partial cellular reprograming in the not so distant future.
To watch the entire conversation clic here: https://youtu.be/QuBo2zMLZ8A
The footage, uploaded to YouTube by local observers, is admittedly sped up between at least two to four times, as Newsweek points out — but the grace at which it moonwalks across the ground in front of it and give a salute is a sight to behold in itself. The robot was finally completed last month, according to Japanese news site SoraNews24. The massive structure weighs over 55,000 pounds and is modeled after the RX-78–2 unit from the popular “Gundam” science fiction franchise.
The robot still hasn’t been revealed to the public, because the ongoing pandemic has indefinitely delayed its opening at the Gundam Factory in the port of Yokohama, Japan. It was originally meant to go on display in October of this year.

Before #COVID19, we like to imagine a #future where we can get and do anything from home, including working, with the help of novel #technologies such as #VR and #AR.
However, the #COVID19 pandemic shows us the human nature, that is, “going out” is one of the basic needs for human being!
One revelation here is that: When speaking of how #technology can change our lives, we often neglect the humane factors and focus only on the technical ones. Take #VR as an example. Yes, it does allow you to have a shopping experience similar to (or even better than) shop outside. However, do you really want to stay at home 24/7 and complete everything online?
https://tek.io/2RP6LGn
by Esther Shein from TechRepublic
#WorkFromHome #FutureOfWork #RemoteWorking
Mental health was the No.1 factor influencing future working environments, the ongoing IBM Institute for Business Value survey found.


While critically ill patients experience a life-threatening illness, they commonly contract ventilator-associated pneumonia. This nosocomial infection increases morbidity and likely mortality as well as the cost of health care. This article reviews the literature with regard to diagnosis, treatment, and prevention. It provides conclusions that can be implemented in practice as well as an algorithm for the bedside clinician and also focuses on the controversies with regard to diagnostic tools and approaches, treatment plans, and prevention strategies.
Patients in the intensive care unit (ICU) are at risk for dying not only from their critical illness but also from secondary processes such as nosocomial infection. Pneumonia is the second most common nosocomial infection in critically ill patients, affecting 27% of all critically ill patients (170). Eighty-six percent of nosocomial pneumonias are associated with mechanical ventilation and are termed ventilator-associated pneumonia (VAP). Between 250,000 and 300,000 cases per year occur in the United States alone, which is an incidence rate of 5 to 10 cases per 1,000 hospital admissions (134, 170). The mortality attributable to VAP has been reported to range between 0 and 50% (10, 41, 43, 96, 161).

A new type of air filter has the potential to work faster, cheaper and better than any other, killing virtually all airborne bacteria and viruses in a fraction of a second.
It’s a germaphobes dream, and a bullish weapon against the spread of infectious diseases, some of which, like measles, can remain suspended in the air for hours on end.
The new device is not only effective at killing viruses, it can actually prevent disease. And if the technology can be miniaturised, the authors say it may one day replace the century-old face mask, allowing us to breathe clean air on the fly.

Las Vegas hosted two successful test flights using unmanned aircraft to carry human organs and tissue last week. On Sept. 17th, MissionGo, a provider of unmanned aviation solutions and Nevada Donor Network, conducted two unmanned flights — one of which was the longest organ delivery flight in Unmanned Aircraft System (UAS) history. The first flight involved transport of research corneas fromSouthern Hills Hospital and Medical Center to Dignity Health — St. Rose Dominican, San Martín Campus.