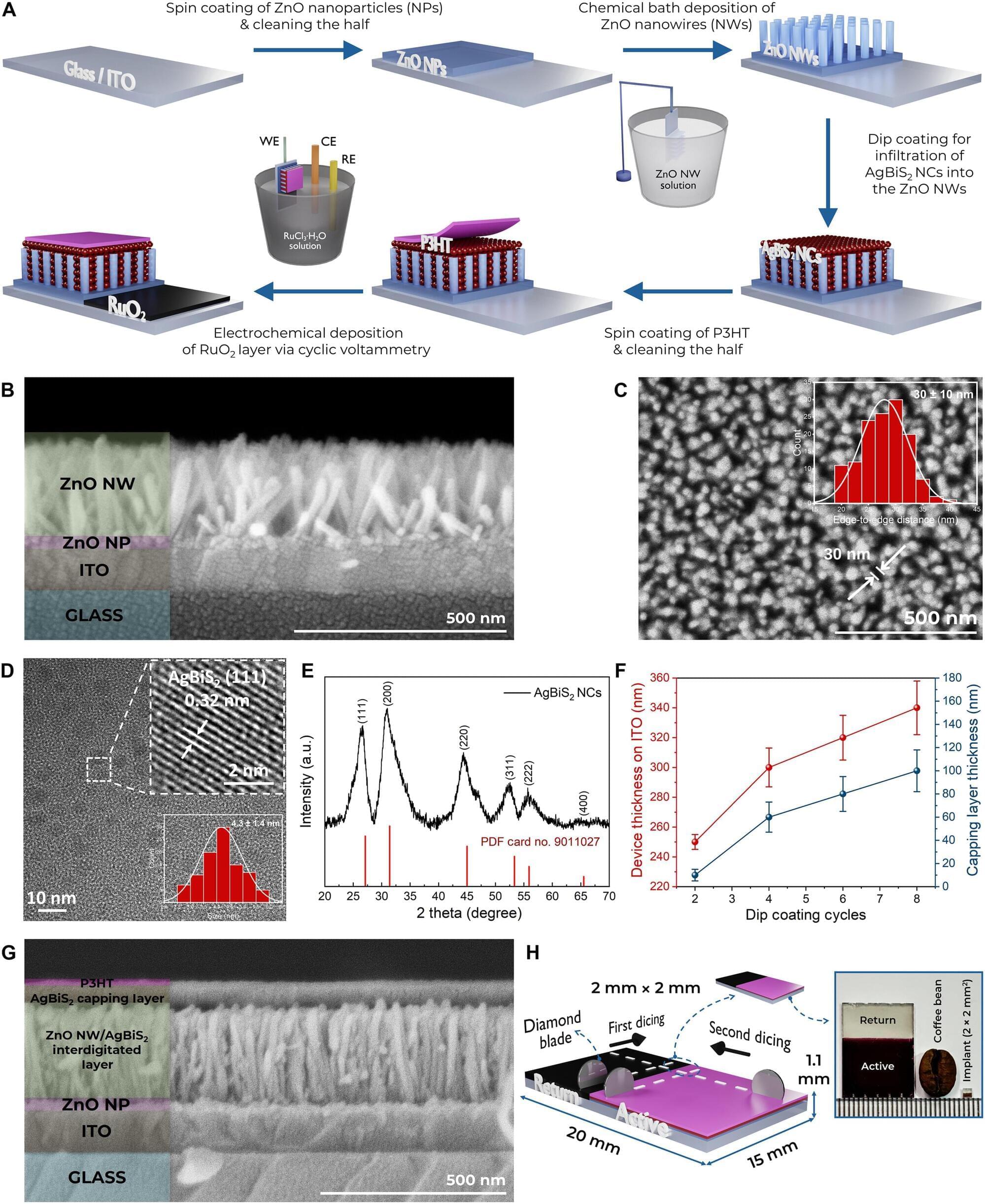
New in eNeuro from Wolfe et al: Brain structures related to shifting between tasks or updating information about the environment show signs of deterioration in late adulthood.
▶️
Cognitive flexibility, a mental process crucial for adaptive behavior, involves multi-scale functioning across several neuronal organization levels. While the neural underpinnings of flexibility have been studied for decades, limited knowledge exists about the structure and age-related differentiation of the white matter subserving brain regions implicated in cognitive flexibility. This study investigated the population-level relationship between cognitive flexibility and properties of white matter across two periods of human adulthood, aiming to discern how these associations vary over different life stages and brain tracts among men and women. We propose a novel framework to study age effects in brain structure-function associations. First, a meta-analysis was conducted to identify neural regions associated with cognitive flexibility. Next, the white matter projections of these neural regions were traced through the Human Connectome Project tractography template to identify the white matter structure associated with cognitive flexibility. Then, a cohort analysis was performed to characterize myelin-related macromolecular features using a subset of the UK Biobank magnetic resonance imaging (MRI) data, which has a companion functional/behavioral dataset. We found that the wiring of cognitive flexibility is defined by a subset of brain tracts, which present undifferentiated features early in adulthood and significantly differentiated types in later life. These MRI-derived properties are correlated with individual subprocesses of cognition, which are closely related to cognitive flexibility function. In late life, myelin-related homogeneity of specific white matter tracts implicated in cognitive flexibility declines with age, a phenomenon not observed in early life. Our findings support the age-related differentiation of white matter tracts implicated in cognitive flexibility as a natural substrate of adaptive cognitive function.
Significance Statement Cognitive flexibility function facilitates adaptation to environmental demands. Brain changes affecting structural organization during the lifespan are theorized to impact cognitive flexibility. This study characterizes how the brain’s connectivity is correlated with cognitive flexibility function throughout adulthood. By analyzing myelin-related properties of white matter, this study found that certain parts of the brain’s wiring related to cognitive flexibility become more differentiated with advanced age. These age-related features appear as a natural characteristic of the human brain that may impact specific aspects of adaptive thinking, like shifting between tasks or updating information.