New research links vitamin D to longevity, cancer risk, and heart health. Learn what studies show about telomeres, inflammation, and aging.



“This Perspective concludes that an MLA between 18–21 years is a scientifically supportable and socially coherent threshold for non-medical cannabis use.”
What should be the minimum legal age for recreational cannabis? This is what a recent study published in The American Journal on Drug and Alcohol Abuse hopes to address as a team of scientists investigated the benefits and challenges of raising the legal age for using recreational marijuana to 25, with the current age range being 18 to 21, depending on the country. This study has the potential to help researchers, legislators, and the public better understand the neuroscience behind the appropriate age for cannabis use.
For the study, the researchers examined brain development for individuals aged 18–25, specifically regarding brain maturation and whether this ceases before age 25. They note it depends on a myriad of factors, including sex, geographic region, and physiology. This study comes as Germany recently published several studies regarding legalizing recreational marijuana nationwide and marijuana use rates post-legalization. In the end, the researchers for this most recent study concluded that raising the minimum legal age for recreational cannabis use to 25 is unnecessary.
The study notes, “This Perspective concludes that an MLA between 18–21 years is a scientifically supportable and socially coherent threshold for non-medical cannabis use. Policy decisions should be informed not only by neurobiological evidence but also by legal, justice, sociocultural, psychological, and historical considerations.”
The age-old advice to “trust your gut” could soon take on new meaning for people diagnosed with Parkinson’s disease, thanks to a creative feat of bioengineering by researchers in the University of Georgia’s College of Veterinary Medicine.
Anumantha Kanthasamy, professor and director of the Isakson Center for Neurological Disease Research (ICNDR) leads a multidisciplinary research team including Gregory Phillips, Piyush Padhi, and other scientists that has engineered a groundbreaking living medicine, a beneficial probiotic designed to deliver levodopa steadily from the gut to the brain of Parkinson’s patients.
In a paper published in the journal Cell Host & Microbe, Kanthasamy’s team details how they engineered and tested the probiotic bacterium Escherichia coli Nissle 1917 as a drug-delivery system that continuously produces and delivers the gold-standard Parkinson’s drug, which is converted to dopamine in the brain. The E. coli Nissle strain was chosen for its century-long record of safely treating gastrointestinal disorders in humans.
As drug-resistant infections continue to rise, researchers are looking for new antimicrobial strategies that are both effective and sustainable. One emerging approach combines nanotechnology with “green” chemistry, using plant extracts instead of harsh chemicals to produce metal oxide nanoparticles.
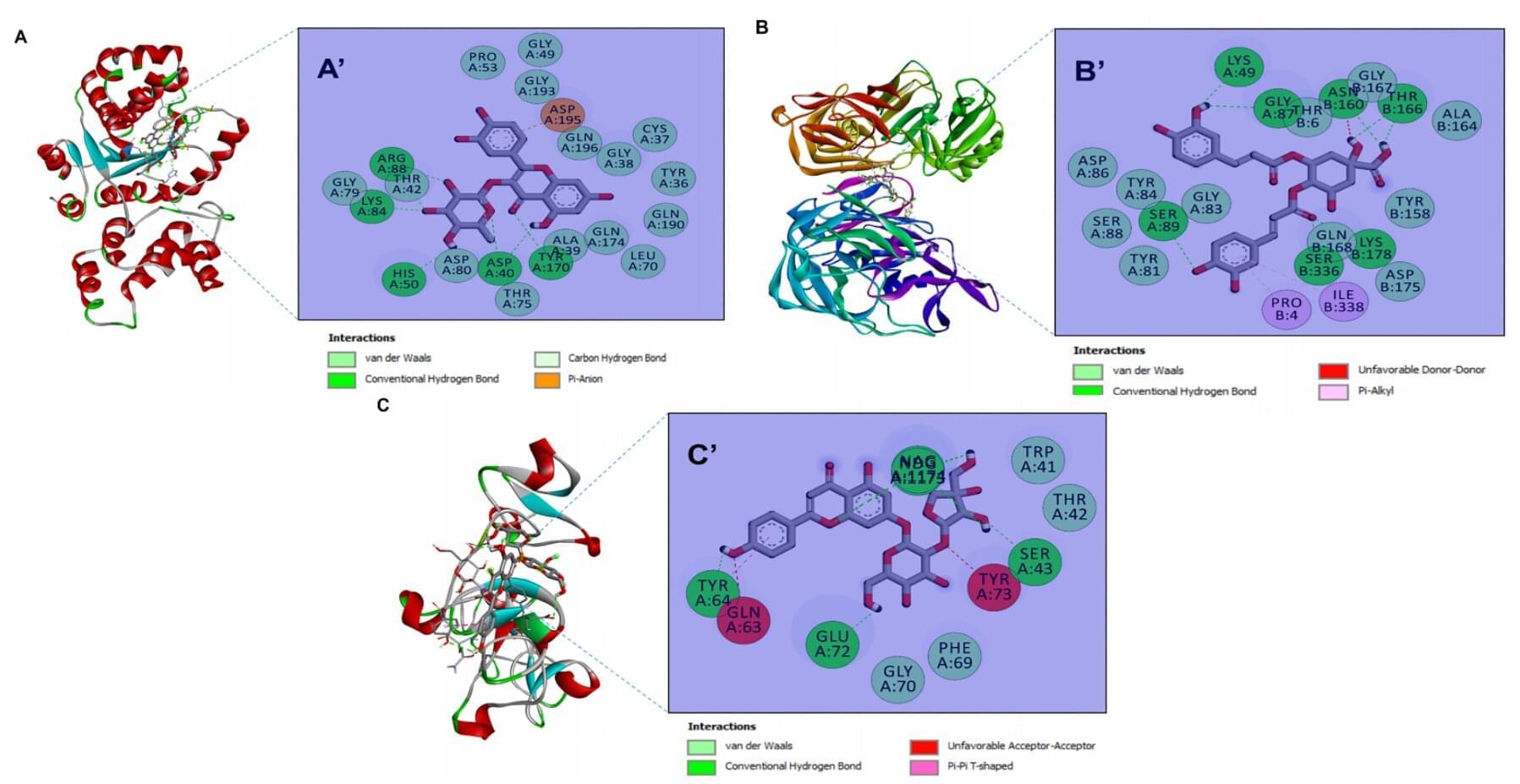
A new study published in Biomolecules and Biomedicine now reports that zinc oxide nanoparticles (ZnONPs) biosynthesized from four desert plants with medicinal properties can inhibit a wide spectrum of bacteria, yeasts and filamentous fungi in laboratory tests. The work also links the plants’ rich phytochemical profiles to nanoparticle stability and potency, and uses computer modeling to explore how key compounds might interact with microbial targets.
The study is the first to produce ZnONPs from species that thrive in harsh, arid environments and are often under-used or even considered invasive. “By turning resilient desert plants into tiny zinc oxide particles, we were able to generate materials that are both eco-friendly to produce and surprisingly active against a range of microbes,” the authors write. “These green nanoparticles could form the basis for future antimicrobial formulations, pending further safety and efficacy testing.”

Ferroelectric materials are used in infrared cameras, medical ultrasounds, computer memory and actuators that turn electric properties into mechanical properties and vice-versa. Most of these essential materials, however, contain lead and can therefore be toxic.
“For the last 10 years, there has been a huge initiative all over the world to find ferroelectric materials that do not contain lead,” said Laurent Bellaiche, Distinguished Professor of physics at the University of Arkansas.
The atoms in a ferroelectric material can have more than one crystalline structure. Where two crystalline structures meet is called a phase boundary, and the properties that make ferroelectric materials useful are strongest at these boundaries.

A hidden physical change in the body may be helping to drive the prolonged malaise some people experience after contracting COVID-19.
Analyzing blood samples from patients with long COVID, a team of medical researchers has identified unusual microscopic structures that may contribute to symptoms such as brain fog and fatigue. If this is the case, it offers a hopeful target for future treatment.
“This study shows a robust association between biomarkers indicative of thromboinflammatory activity and long COVID,” the team writes in a paper led by geneticist Alain Thierry of Montpellier University in France.
In a major step forward for cancer care, researchers at ChristianaCare’s Gene Editing Institute have shown that disabling the NRF2 gene with CRISPR technology can reverse chemotherapy resistance in lung cancer. The approach restores drug sensitivity and slows tumor growth. The findings are published in the journal Molecular Therapy Oncology.
This breakthrough stems from more than a decade of research by the Gene Editing Institute into the NRF2 gene, a known driver of treatment resistance. The results were consistent across multiple in vitro studies using human lung cancer cell lines and in vivo animal models.
“We’ve seen compelling evidence at every stage of research,” said Kelly Banas, Ph.D., lead author of the study and associate director of research at the Gene Editing Institute. “It’s a strong foundation for taking the next step toward clinical trials.”

University of Kentucky researchers have developed a new experimental model that could point the way toward more effective Alzheimer’s disease treatments by targeting one of the brain’s most important genes for risk and resilience.
The study, published in Nature Neuroscience, focuses on apolipoprotein E (APOE), a gene long known to play a major role in Alzheimer’s disease. The team created a first-of-its-kind mouse model that allows scientists to “flip a switch,” changing the high-risk version of the gene (APOE4) to the protective form (APOE2) in adult animals.

Lipid nanoparticles (LNPs) have emerged as popular vehicles for delivering various types of drugs such as mRNA and gene therapy. While these nanoparticles are effective in transporting therapeutic payloads, their components can interact with the human body, potentially causing genotoxicity — damage to the recipient’s genetic material that may lead to inheritable mutations or cancer. In this webinar brought to you by Inotiv, Shambhu Roy will discuss how to test the genotoxicity of LNP-based therapeutics to ensure the safety of these innovative drug delivery systems.
We’ll chat about these topics.
• Understanding the key components of LNP delivery systems • Genotoxicity testing for LNP-based drugs during preclinical safety assessment • Selecting the appropriate assays to meet regulatory requirements.
Although the flu season seems off to a quiet start in the US, warnings from abroad suggest that a new strain may yet shake up the US healthcare system. New data reported in the British Medical Journal showed a significant increase in flu rates in the UK, largely among young adults and school-aged children. The cases are dominated by a new H3N2 strain, which mutated several times over the summer, increasing in severity, according to experts.
Data from Canada, UK, and Japan reveal an H3N2 flu variant that may affect 2025–2026 season.