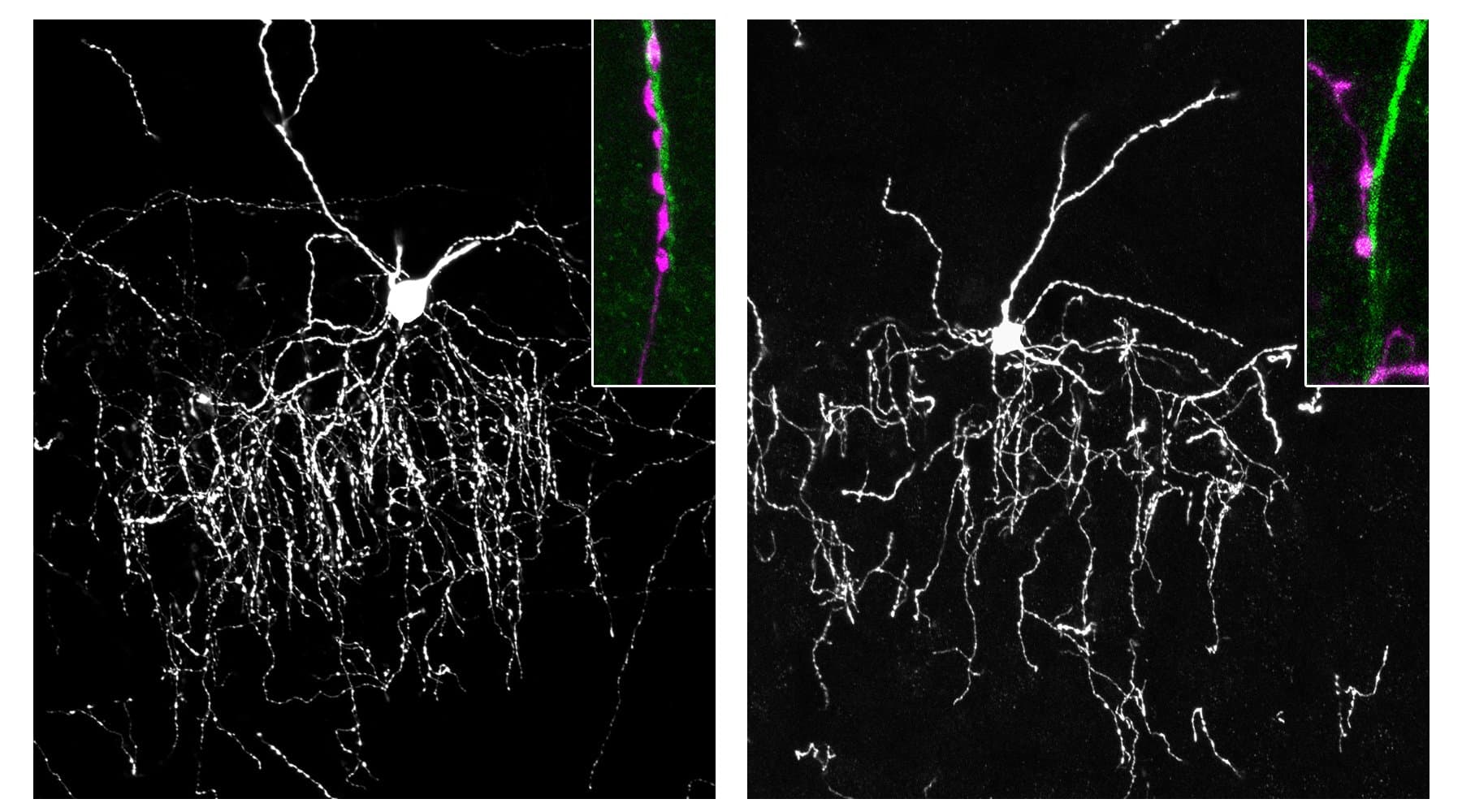
A study of 155 adults found that the correlation between inflammatory markers and symptoms like anxiety or fatigue is significantly more pronounced in individuals with low sleep quality or difficulty regulating emotions.



Is it possible to spot personality dysfunction from someone’s everyday word use? My colleagues and I have conducted research that suggests you can, and often sooner than you might expect.
Whether in a quick text message, a long email, a casual chat with a friend, or a comment online, the words people choose quietly reveal deeper patterns in how they think, feel, and relate to others.
Everyone has personality traits – habitual ways of thinking, feeling and behaving. When these patterns become rigid, intense or disruptive, they can cause ongoing problems with emotions, sense of self and relationships.

A new study published in the journal Personality and Individual Differences shows that men with higher general intelligence are less likely to engage in abusive or coercive behaviors toward their romantic partners. The findings suggest that cognitive ability may play a role in how men manage conflict and commitment in heterosexual relationships.
General intelligence is a broad mental capacity that influences reasoning, planning, and problem-solving. Psychology research has long established that people with higher general intelligence tend to experience better life outcomes. They generally achieve higher levels of education and earn more money. They also tend to live longer and suffer from fewer health issues.
But the relationship between intelligence and romantic success is less clear. Some data suggests that intelligent people are less likely to divorce. Other studies indicate they may have sex less frequently or choose to have fewer children. Evolutionary psychologists have debated why this might be the case.