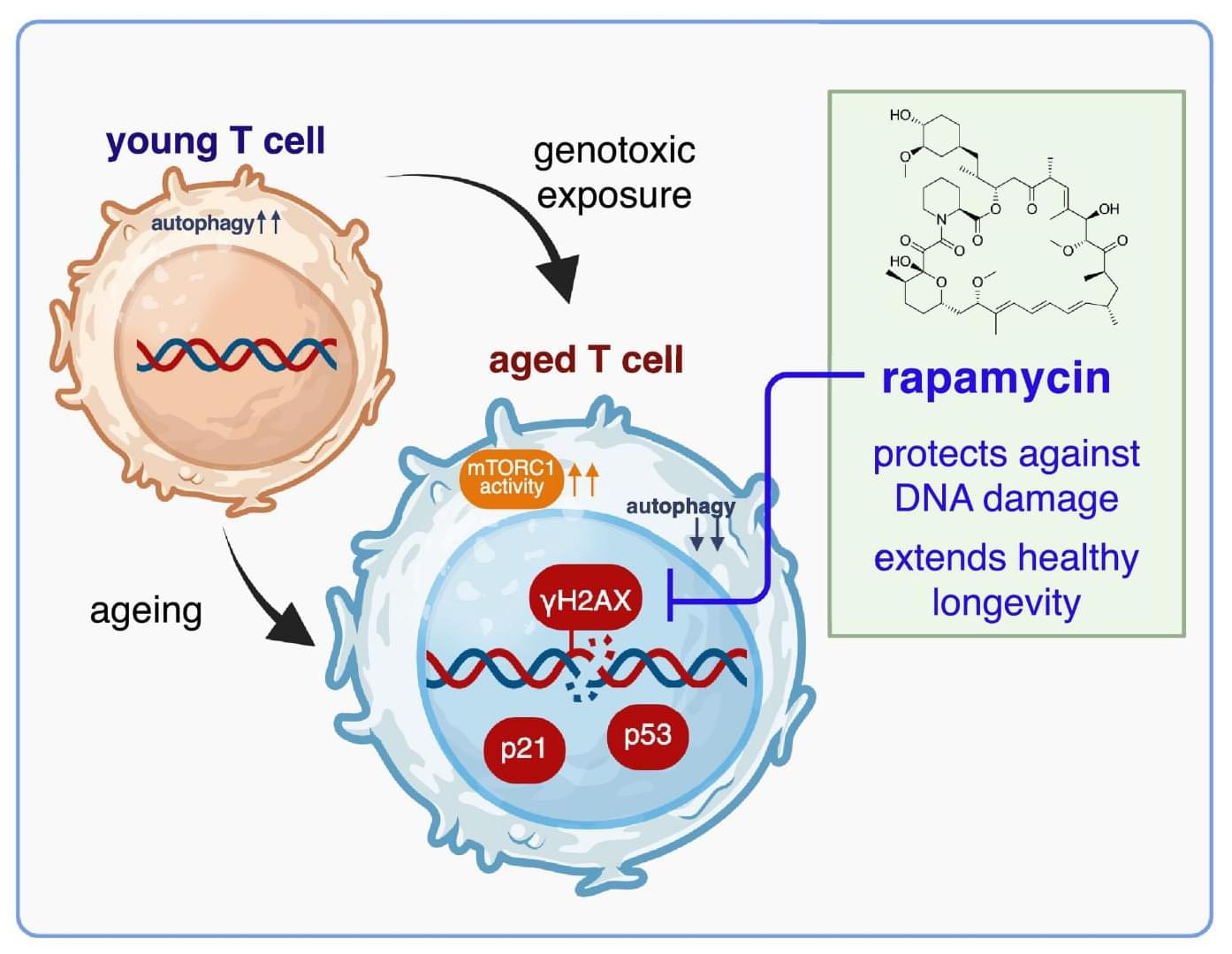
Using in vitro DNA damage assays in human T cells, ex vivo profiling of aged immune subsets and a small placebo-controlled in vivo study, authors show that low-dose rapamycin, a potent life-extending…

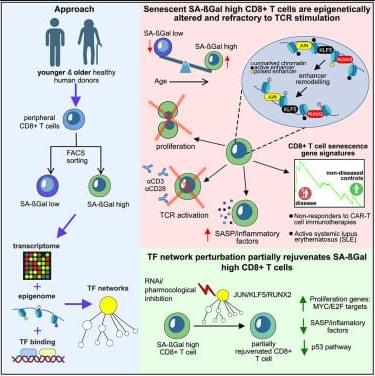
Turano et al. reveal the transcription factor networks driving CD8+ T cell senescence in healthy aging humans. Inhibiting key transcription factors modulates the senescence program and partially restores responsiveness to TCR stimulation. They also show that CD8+ T cell senescence gene signatures predict response to CAR-T cell therapy in B cell lymphomas.
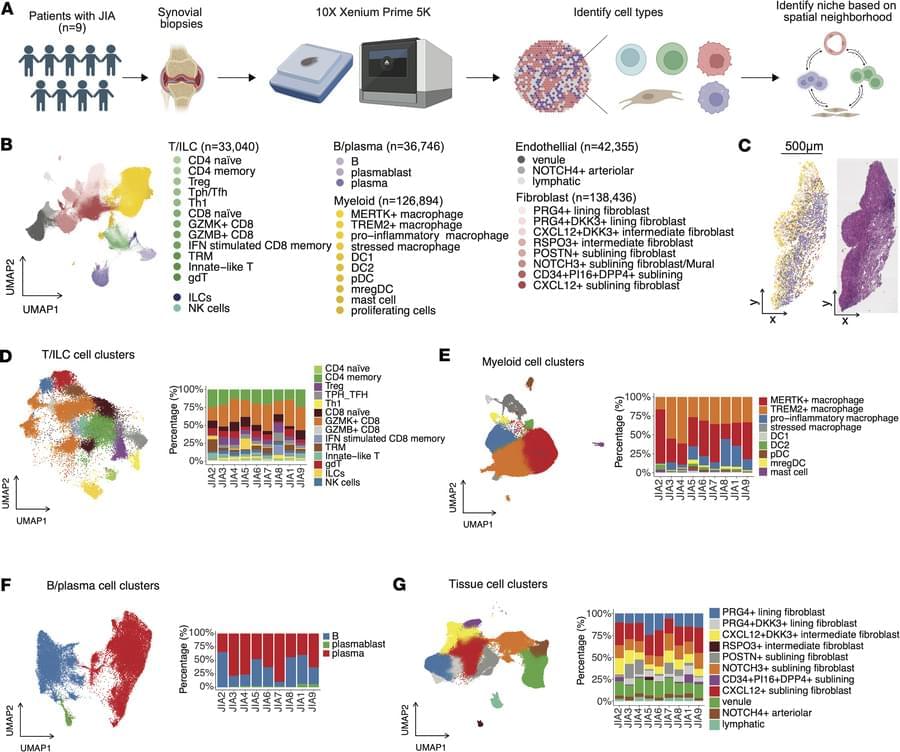
Jun Inamo & team profiles nearly 400,000 cells across 9 patients with juvenile idiopathic arthritis (JIA), revealing disease-specific immune–stromal interactions within the synovium.
1Department of Biomedical Informatics, Center for Health Artificial Intelligence, and.
2Division of Rheumatology, University of Colorado School of Medicine, Aurora, Colorado, USA.
3Division of Rheumatology, Department of Internal Medicine, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan.



Sarcoidosis is a systemic, granulomatous disorder commonly affecting the lungs that has the potential to cause numerous thoracic complications. We present a novel case of a 44-year-old woman with pulmonary sarcoidosis who demonstrated a large pulmonary infarction. The disease presentation ultimately was attributed to arterial stenosis resulting from sarcoidosis-associated fibrosing mediastinitis and compressive mediastinal adenopathy. The patient was treated with an extended course of prednisone and subsequently was transitioned to azathioprine with eventual resolution of symptoms, but persistence of imaging findings.

With the rise of AGI, the need for Primal eye theory and the understanding of our form of sentience becomes greater than ever if we who are “Born of Nature” are to remain relevant.
Which is, of course, my doing. I’ve been working on a couple of big projects across the summer; time has been, to say the least, at a premium. The real point is: both projects have major implications for the future of this newsletter. I can’t wait to share more on all that in the coming weeks.
In the meantime, though, I’ve still been writing a whole lot. As most of you know, along with Raoul Pal I run The Exponentialist, a community focused on emerging technologies and their economic, social, and human implications.
In this special update, then, I’d like to share a recent essay from The Exponentialist. One that allows you a glimpse of the kind of work I do there. And that articulates an set of idea that are at the heart of my current thinking when it comes to our journey into the decades ahead.

Enzymes are the molecular machines that power life; they build and break down molecules, copy DNA, digest food, and drive virtually every chemical reaction in our cells. For decades, scientists have designed drugs to slow down or block enzymes, stopping infections or the growth of cancer by jamming these tiny machines. But what if tackling some diseases requires the opposite approach?
Speeding enzymes up, it turns out, is much harder than stopping them. Tarun Kapoor is the Pels Family Professor in Rockefeller’s Selma and Lawrence Ruben Laboratory of Chemistry and Cell Biology. Recently, he has shifted the focus of this lab to tackle the tricky question of how to make enzymes work faster.
Already, his lab has developed a chemical compound to speed up an enzyme that works too slowly in people with a rare form of neurodegeneration. The same approach could open new treatment possibilities for many other diseases where other enzymes have lost function, including some cancers and neurodegenerative disorders such as Alzheimer’s.

