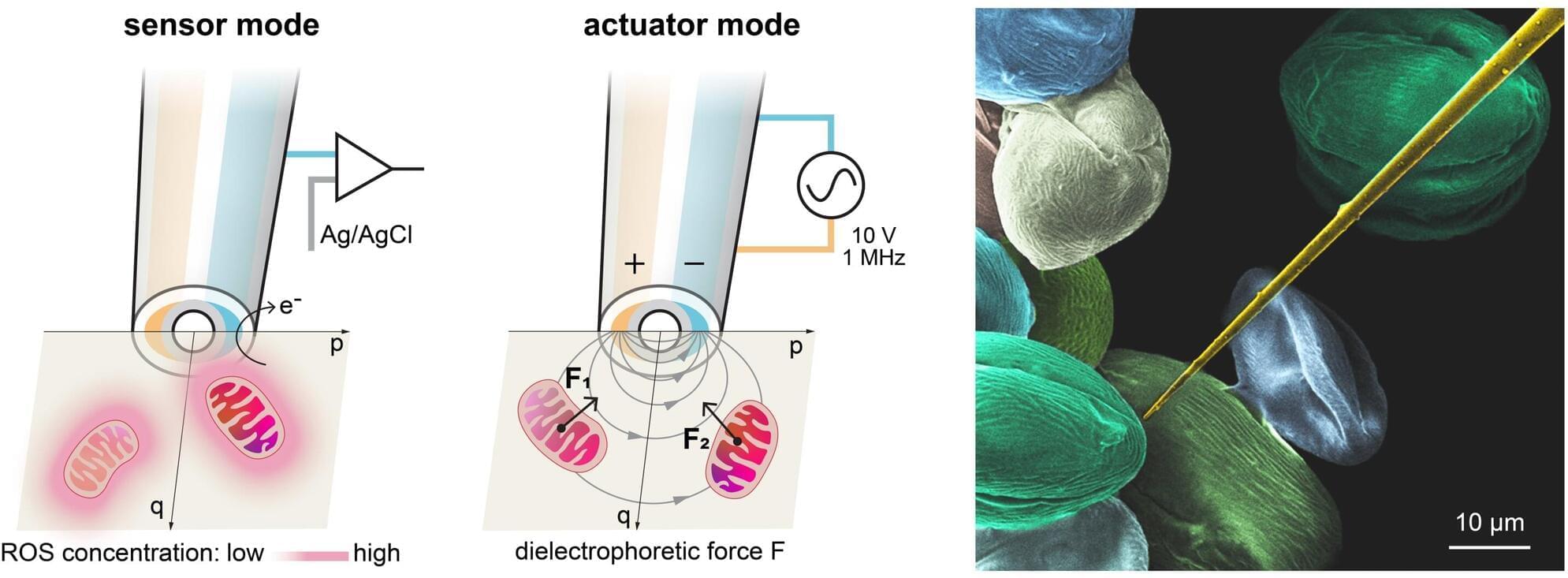
Recent advances in the field of artificial intelligence (AI) have opened new exciting possibilities for the rapid analysis of data, the sourcing of information and the generation of use-specific content. To run AI models, current hardware needs to continuously move data from internal memory components to processors, which is energy-intensive and can increase the time required to tackle specific tasks.
Over the past few years, engineers have been trying to develop new systems that could overcome this limitation, running AI algorithms more reliably and efficiently. One proposed solution is the development of in-memory computing systems.
Content-addressable memory (CAM) is one of the earliest in-memory computing hardware systems, where memory components search for stored data faster, comparing each stored entry simultaneously based on its content, but faces challenges for AI applications because of the fundamental limitation of silicon transistors.