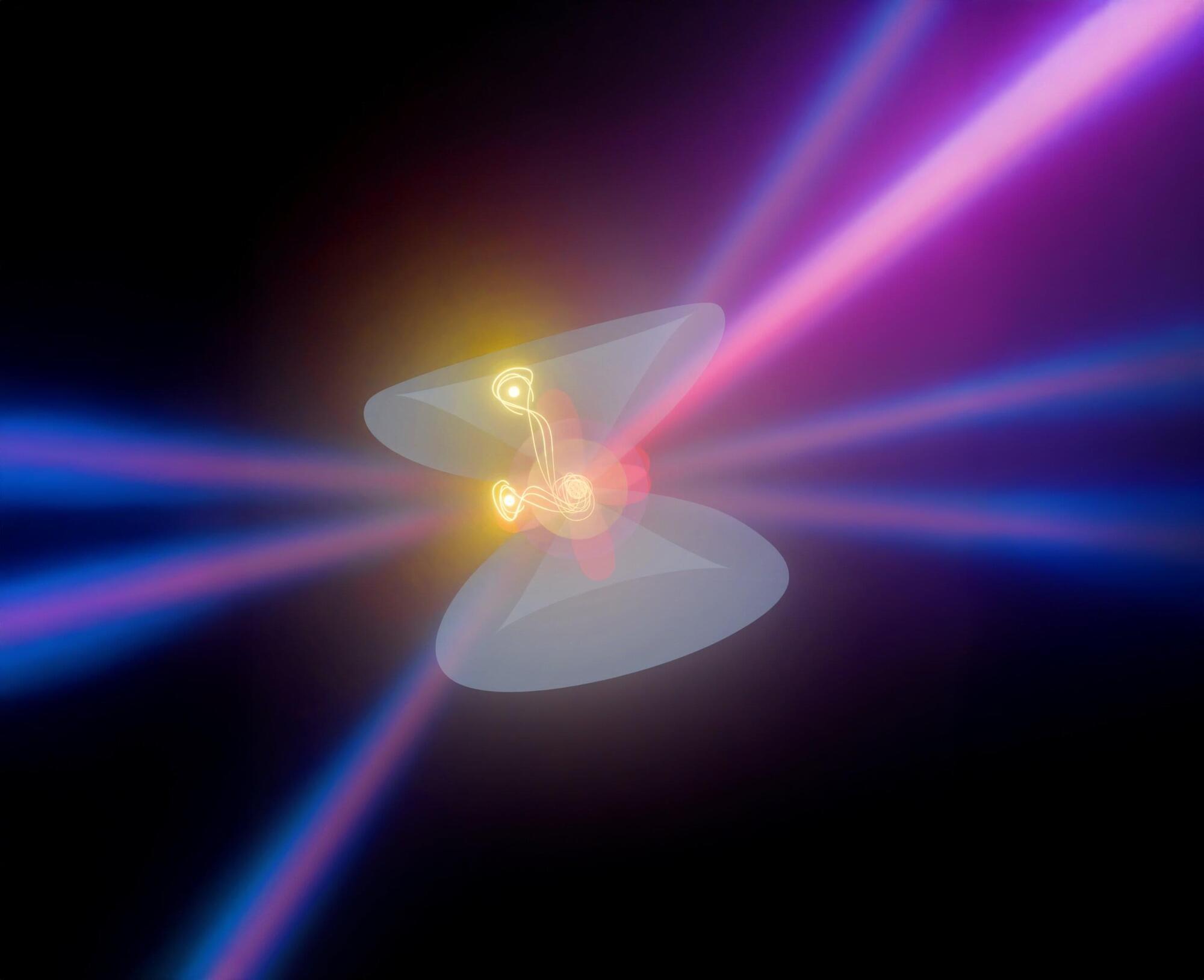
An international team of astronomers, including researchers from the Department of Physics at The University of Hong Kong (HKU), has uncovered the first decisive evidence that at least some fast radio burst (FRB) sources—brief but powerful flashes of radio waves from distant galaxies—reside in binary stellar systems. This means the FRB source is not an isolated star, as previously assumed, but part of a binary stellar system in which two stars orbit each other.
Using the Five-hundred-meter Aperture Spherical Telescope (FAST) located in Guizhou, also known as the “China Sky Eye,” the team detected a distinctive signal that reveals the presence of a nearby companion star orbiting the FRB source.
The discovery, published in Science, is based on nearly 20 months of monitoring an active repeating FRB located about 2.5 billion light-years away.