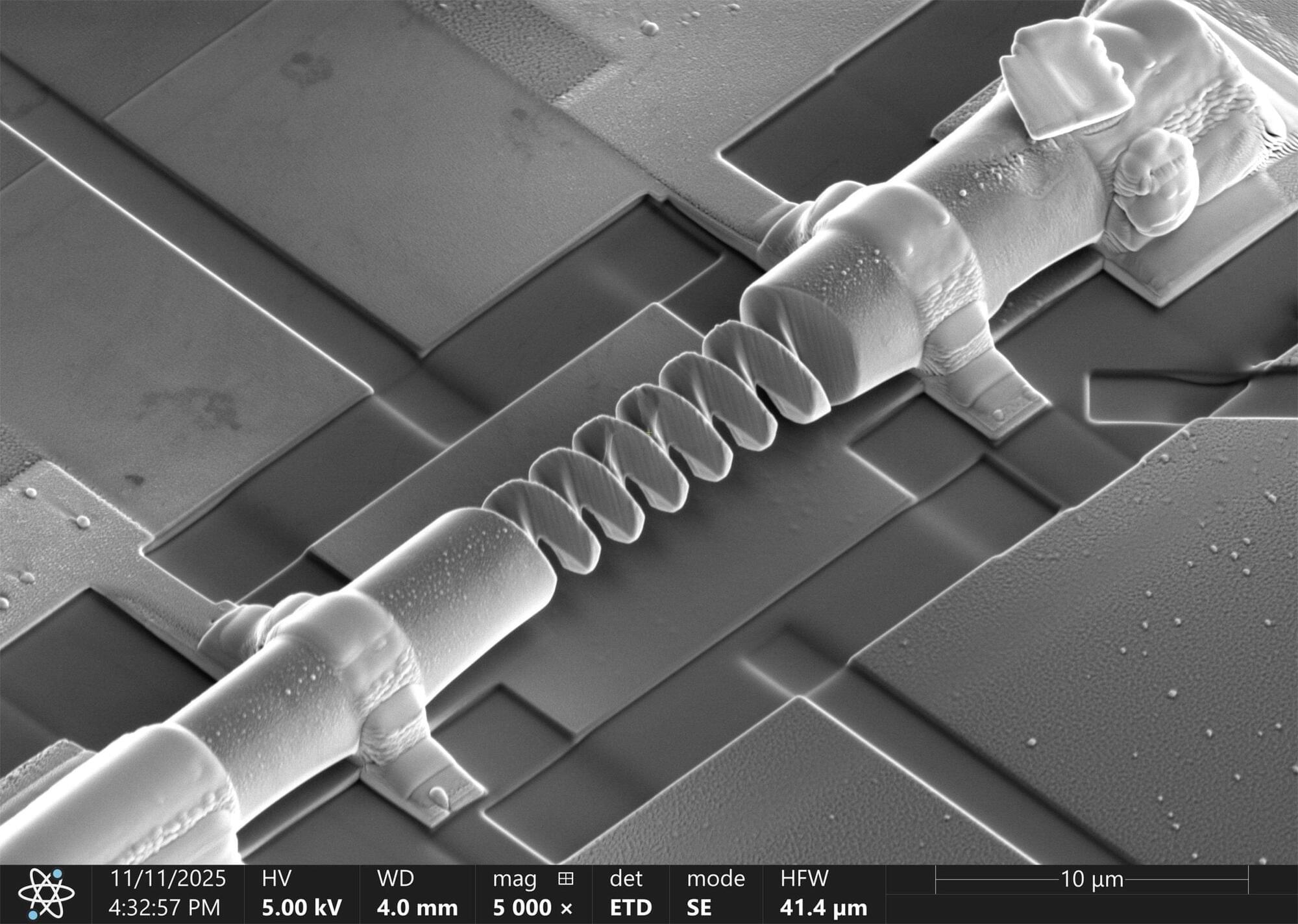
Scientists have shown that twisting a crystal at the nanoscale can turn it into a tiny, reversible diode, hinting at a new era of shape-engineered electronics.

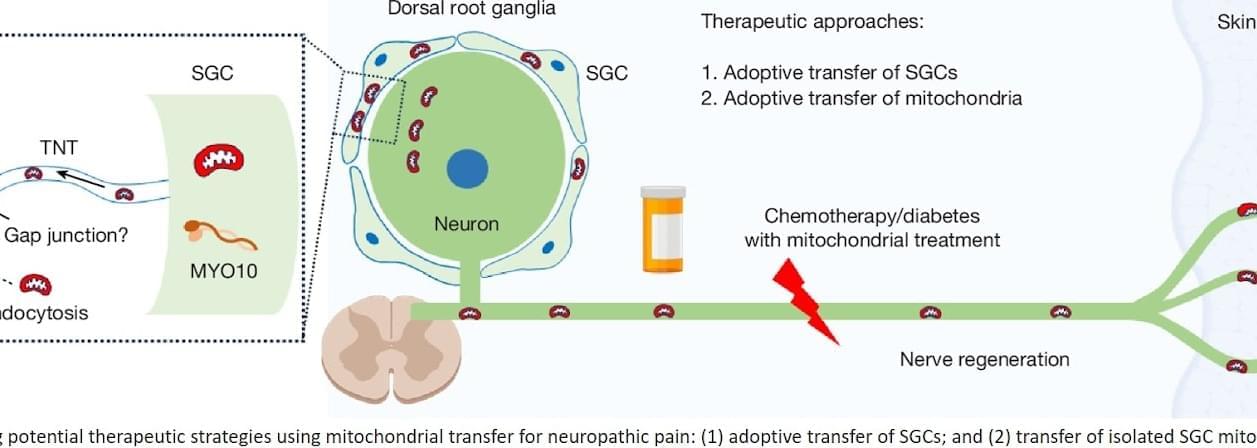
For millions living with nerve pain, even a light touch can feel unbearable. Scientists have long suspected that damaged nerve cells falter because their energy factories known as mitochondria don’t function properly.
Now research published in Nature suggests a way forward: supplying healthy mitochondria to struggling nerve cells.
Using human tissue and mouse models, researchers found that replenishing mitochondria significantly reduced pain tied to diabetic neuropathy and chemotherapy-induced nerve damage. In some cases, the relief lasted up to 48 hours.
Instead of masking symptoms, the approach could fix what the team sees as the root problem — restoring the energy flow that keeps nerve cells healthy and resilient.
“By giving damaged nerves fresh mitochondria — or helping them make more of their own — we can reduce inflammation and support healing,” said the study’s senior author. “This approach has the potential to ease pain in a completely new way.
The work highlights a previously undocumented role for satellite glial cells, which appear to deliver mitochondria to sensory neurons through tiny channels called tunnelling nanotubes.
When this mitochondrial handoff is disrupted, nerve fibers begin to degenerate — triggering pain, tingling and numbness, often in the hands and feet, the distal ends of the nerve fibers.
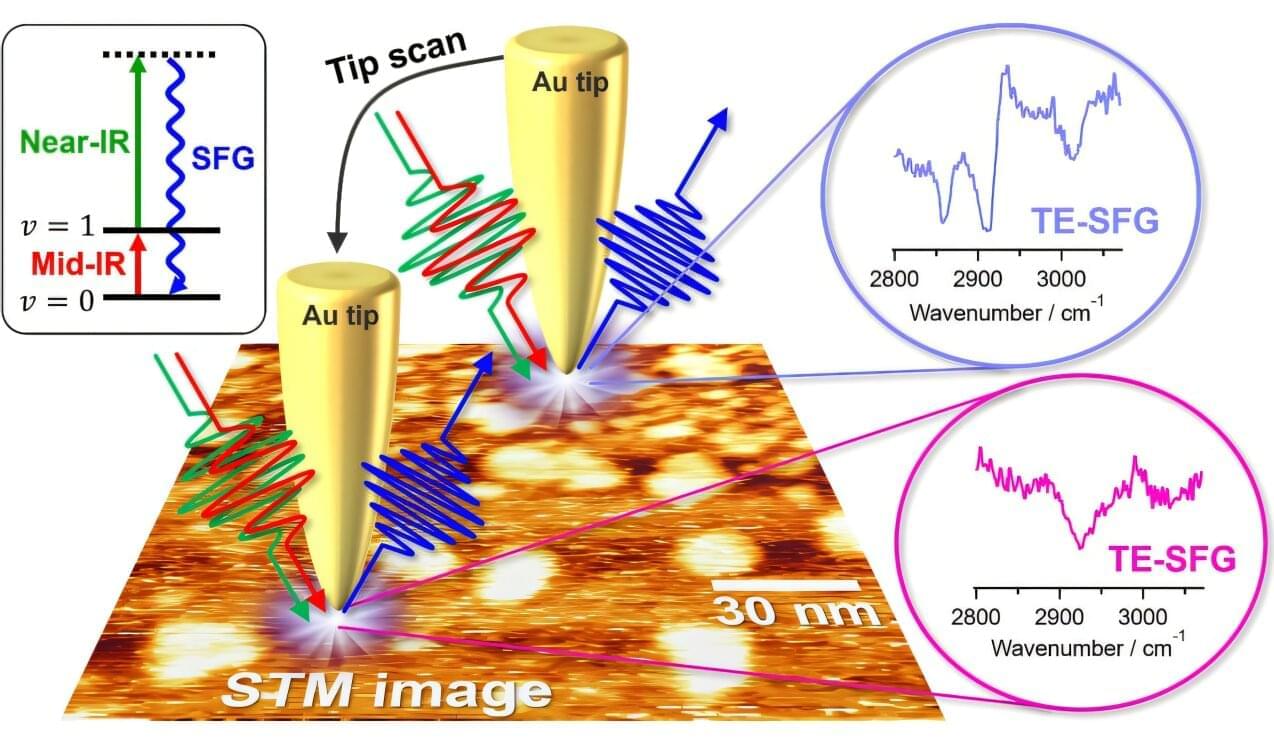
Sum-frequency generation (SFG) is a powerful vibrational spectroscopy that can selectively probe molecular structures at surfaces and interfaces, but its spatial resolution has been limited to the micrometer scale by the diffraction limit of light.
In a study published in The Journal of Physical Chemistry C, investigators overcame this limitation by utilizing a highly confined near field within a plasmonic nanogap and successfully extended the SFG spectroscopy into a nanoscopic regime with ~10-nm spatial resolution.
The team also established a comprehensive theoretical framework that accurately describes the microscopic mechanisms of this near-field SFG process. These experimental and theoretical achievements collectively represent a groundbreaking advancement in near-field second-order nonlinear nanospectroscopy, enabling direct access to correlated chemical and topographic information of interfacial molecular systems at the nanoscale.
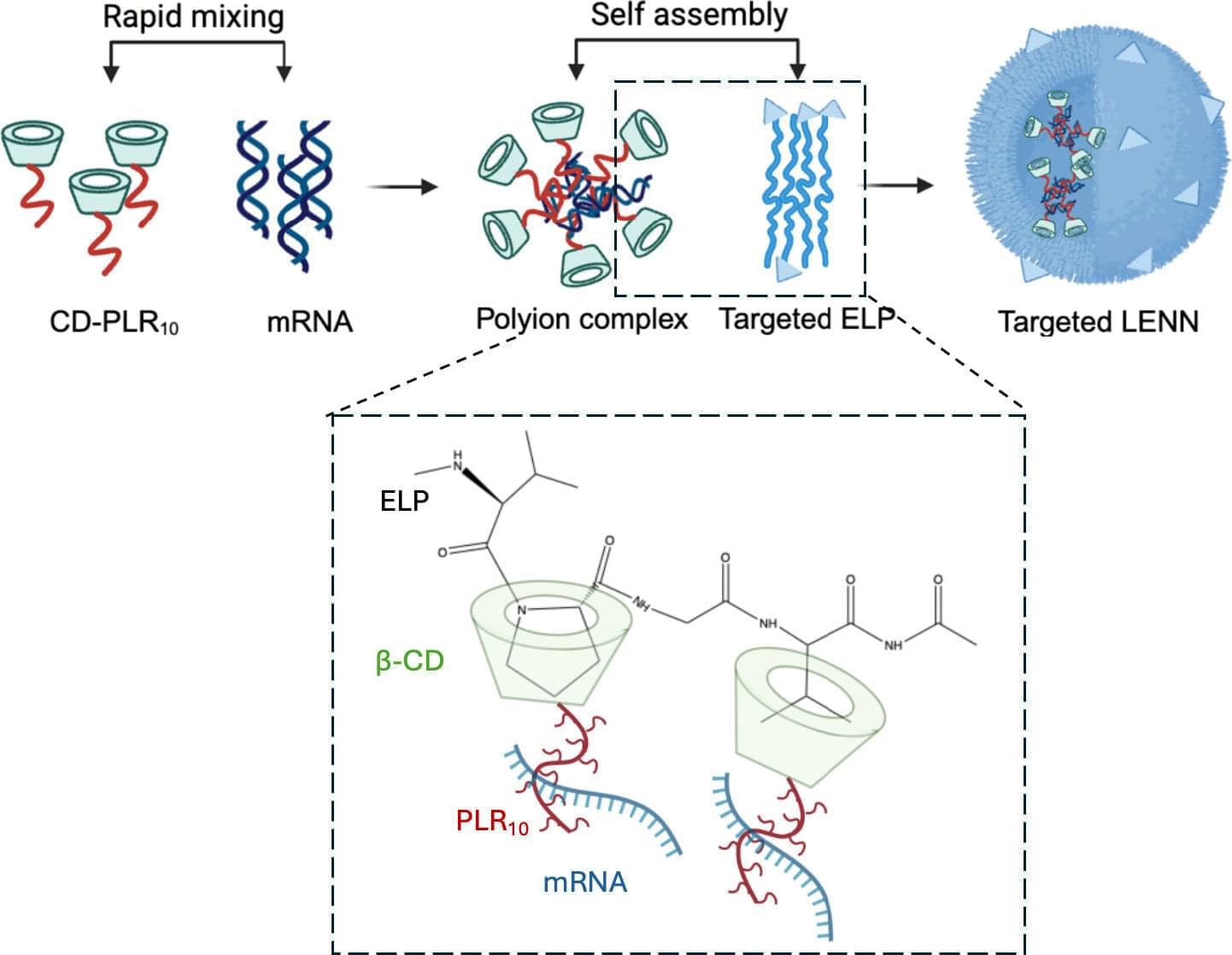
Published, peer-reviewed research shows a patent-pending, virus-mimicking platform technology developed at Purdue University improves upon traditional methods of targeting bladder cancer cells with messenger RNA (mRNA) therapies.
The study, published in the Proceedings of the National Academy of Sciences, highlights compelling features of the therapy-delivering system with respect to size, targetability, encapsulation efficiency, complex stability, gene expression and “green” manufacturability.
David Thompson led the team conducting research about layer-by-layer elastin-like polypeptide nucleic acid nanoparticle (LENN). He is a professor in the James Tarpo Jr. and Margaret Tarpo Department of Chemistry and a member of the Purdue Institute for Cancer Research and the Purdue Institute for Drug Discovery. Saloni Darji, a commercialization postdoctoral research associate, is the paper’s lead author.

Hematopoietic aging extends far beyond the confines of the bone marrow, functioning as a central regulator of systemic decline through its influence on inflammation, immune dysregulation, and inter-organ communication. Moreover, reciprocal signaling from peripheral organs, such as the brain and gut, further shapes hematopoietic aging, highlighting the bidirectional nature of these interactions (Figure 3).
The Intelligence Revolution: Coupling AI and the Human Brain.
New videos DAILY: https://bigth.ink.
Join Big Think Edge for exclusive video lessons from top thinkers and doers: https://bigth.ink/Edge.
Edward Boyden is a Hertz Foundation Fellow and recipient of the prestigious Hertz Foundation Grant for graduate study in the applications of the physical, biological and engineering sciences. A professor of Biological Engineering and Brain and Cognitive Sciences at MIT, Edward Boyden explains how humanity is only at its infancy in merging with machines. His work is leading him towards the development of a “brain co-processor”, a device that interacts intimately with the brain to upload and download information to and from it, augmenting human capabilities in memory storage, decision making, and cognition. The first step, however, is understanding the brain on a much deeper level. With the support of the Fannie and John Hertz Foundation, Ed Boyden pursued a PhD in neurosciences from Stanford University.
EDWARD BOYDEN:
Edward Boyden is a professor of Biological Engineering and Brain and Cognitive Sciences at the MIT Media Lab and the McGovern Institute for Brain Research at MIT. He leads the Media Lab’s Synthetic Neurobiology group, which develops tools for analyzing and repairing complex biological systems, such as the brain, and applies them systematically both to reveal ground truth principles of biological function and to repair these systems.
These technologies, often created in interdisciplinary collaborations, include expansion microscopy (which enables complex biological systems to be imaged with nanoscale precision) optogenetic tools (which enable the activation and silencing of neural activity with light,) and optical, nanofabricated, and robotic interfaces (which enable recording and control of neural dynamics).
Boyden has launched an award-winning series of classes at MIT, which teach principles of neuroengineering, starting with the basic principles of how to control and observe neural functions, and culminating with strategies for launching companies in the nascent neurotechnology space. He also co-directs the MIT Center for Neurobiological Engineering, which aims to develop new tools to accelerate neuroscience progress.
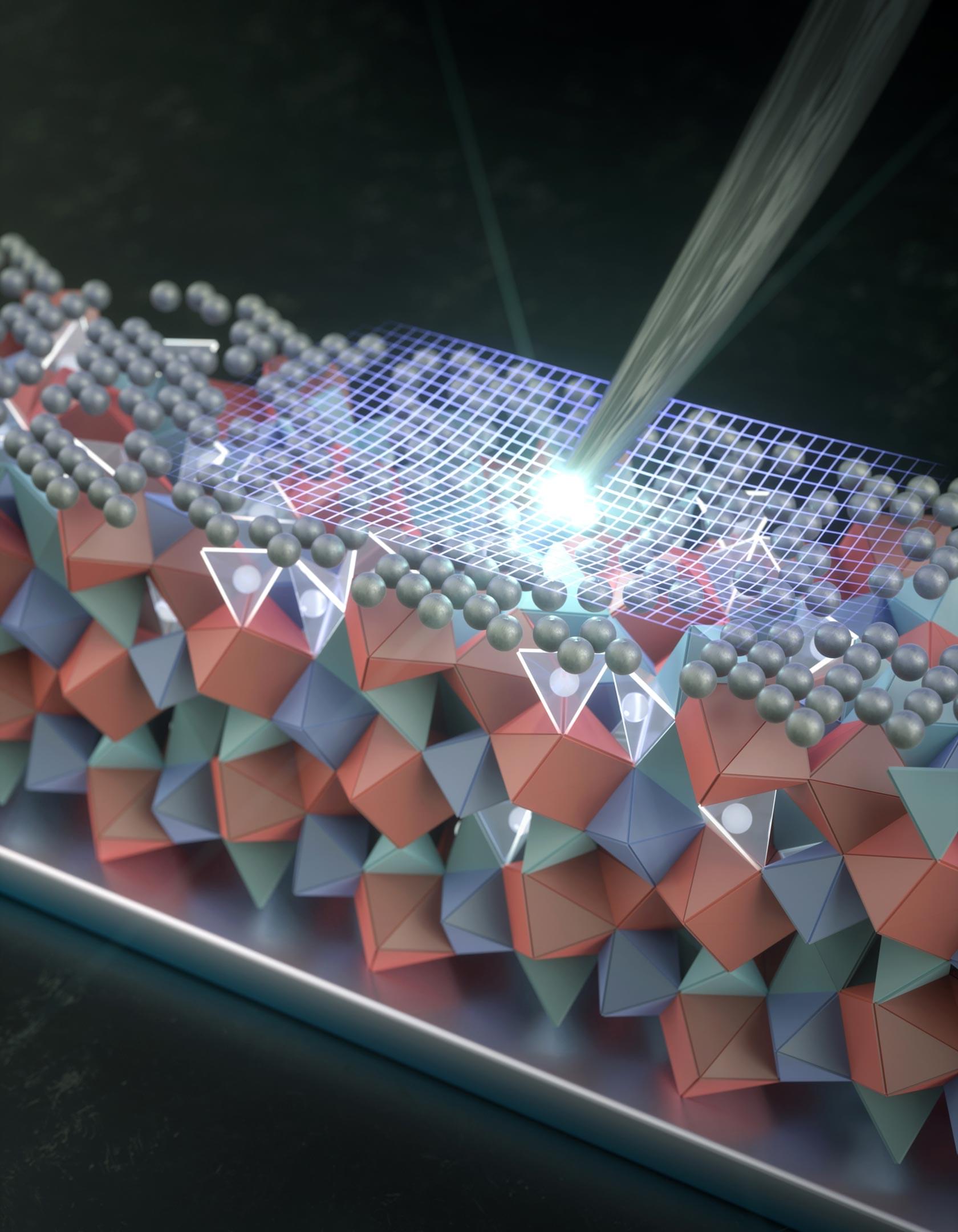
A nanoscale silver coating could be the key to making ultra-powerful solid-state batteries finally work.
Replacing the liquid electrolyte inside today’s batteries with a solid one could unlock a new generation of rechargeable lithium metal batteries. In theory, these batteries would be safer, store far more energy, and recharge much faster than the lithium-ion batteries now in widespread use. Scientists and engineers have been chasing this goal for decades, but progress has been slowed by a persistent flaw. Solid, crystal-based electrolytes tend to develop microscopic cracks that gradually spread during repeated charging and use, eventually causing the battery to fail.
A thin silver layer with a big impact.
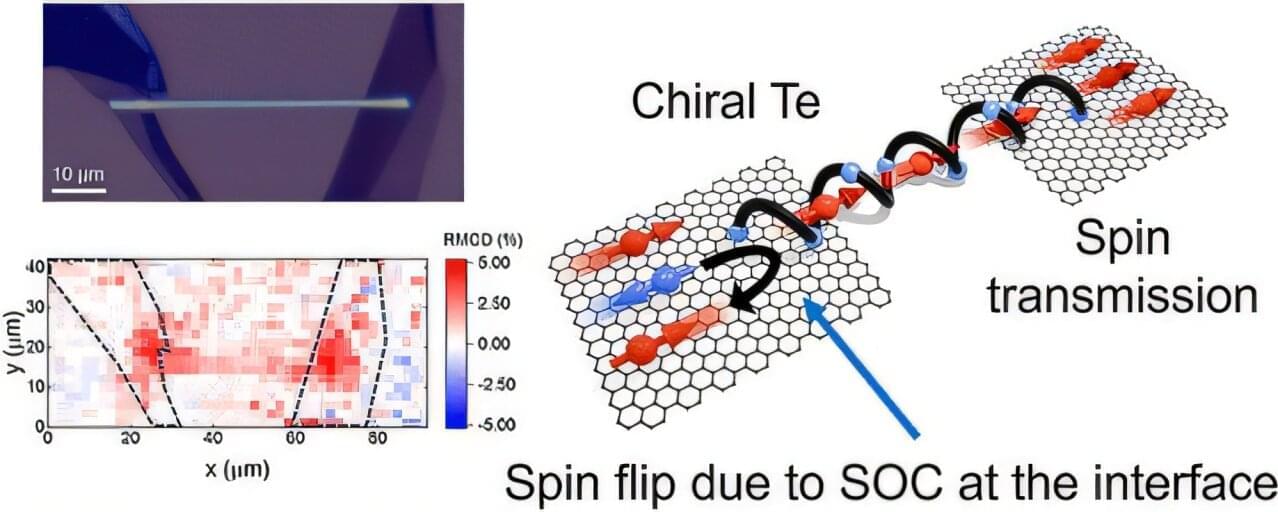
The phenomenon where electron spins align in a specific direction after passing through chiral materials is a cornerstone for future spin-based electronics. Yet, the precise process behind this effect has remained a mystery—until now.
An international team of researchers, affiliated with UNIST, has directly observed how electron spins behave in real space, providing a fresh understanding of this complex interaction. The findings were published in ACS Nano.
Professors Noejung Park and Seon Namgung from the Department of Physics at UNIST, in collaboration with Professor Binghai Yan from Pennsylvania State University, conducted the study. Their work confirms that chiral materials actively change the spin orientation of electrons, overturning the long-held belief that these materials simply filter spins without affecting their direction.
The idea never died, progress is still being made.
Nanotechnology was once imagined as the next great technological revolution—atom-by-atom manufacturing, machines as small as cells, and materials we can only dream of today. Instead, it stalled. While AI, robotics, and nuclear surged ahead, nanotech faded into the background, reduced to buzzwords and sci-fi aesthetics.
But the idea never died.
We can manipulate matter at the atomic scale. We can design perfect materials. We can build molecular machines. What’s been missing isn’t physics—it’s ambition, investment, and the will to push beyond today’s tools.
In this interview with futurist J. Storrs Hall, we explore what nanotechnology really is, why it drifted off course, and why its future may finally be on the horizon. If AI was a “blue-sky fantasy” until suddenly it wasn’t, what happens when someone decides nanotech deserves the same surge of talent, money, and imagination?