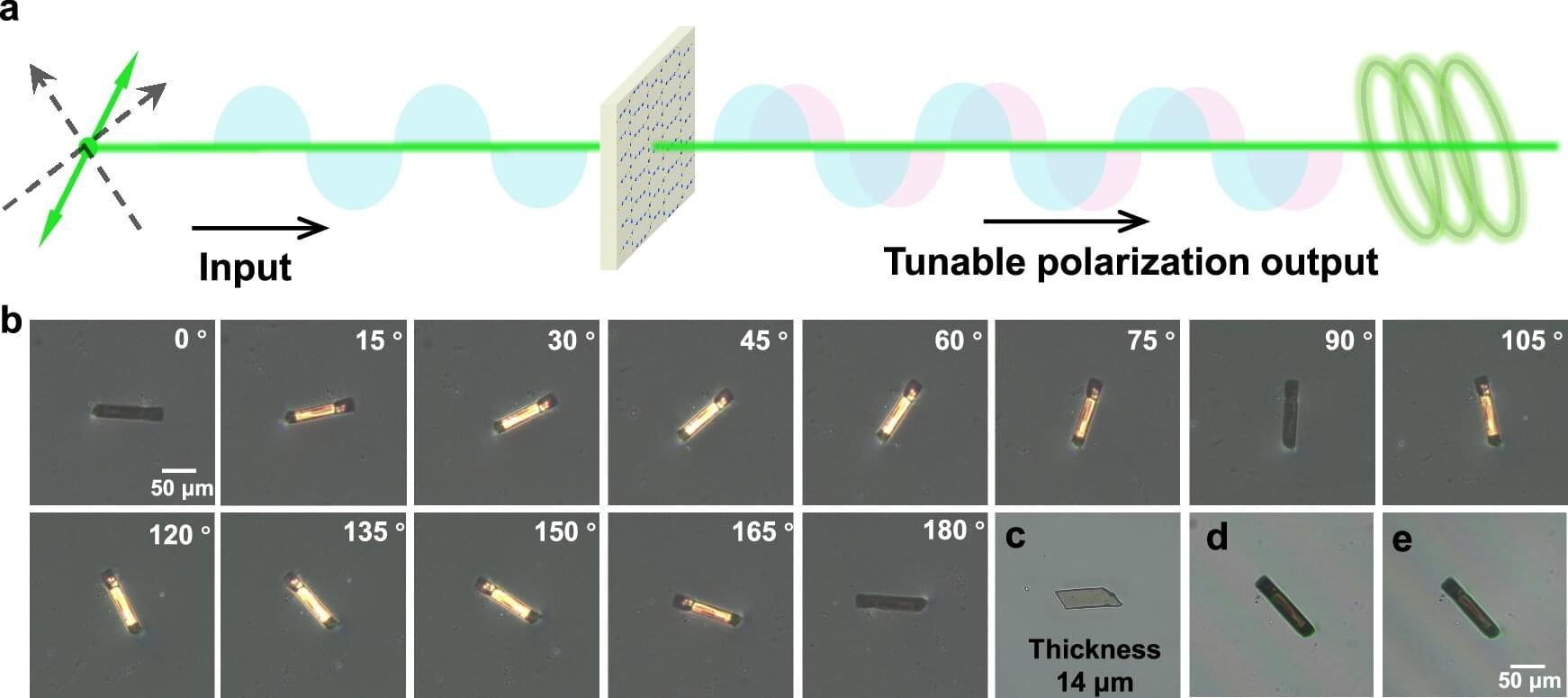
Researchers at the University of Basel and the ETH in Zurich have succeeded in changing the polarity of a special ferromagnet using a laser beam. In the future, this method could be used to create adaptable electronic circuits with light.
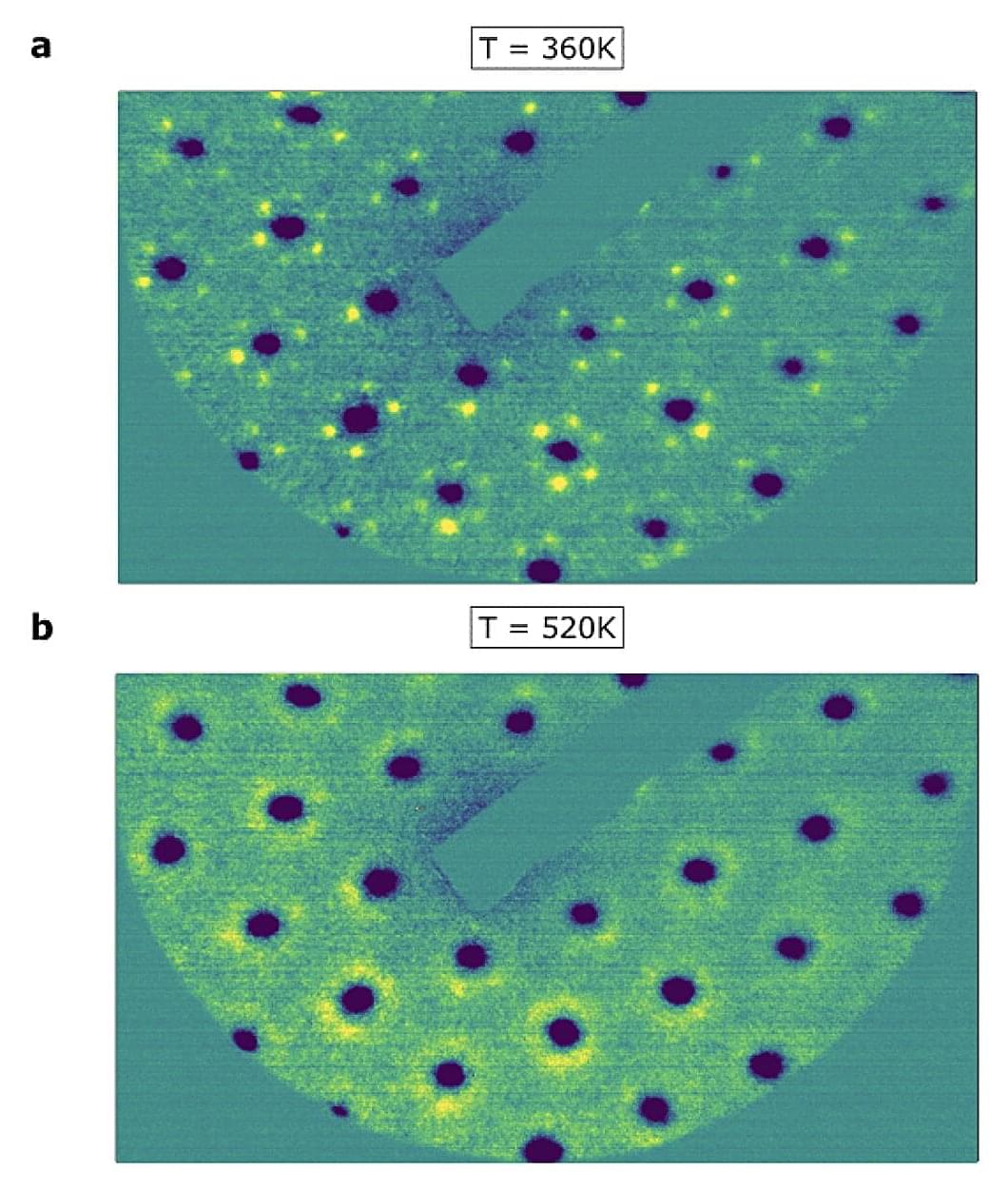
In a ferromagnet, combined forces are at work. In order for a compass needle to point north or a fridge magnet to stick to the fridge door, countless electrons spin inside them, each of which only creates a tiny magnetic field, all need to line up in the same direction. This happens through interactions between the spins, which have to be stronger than the disordered thermal motion inside the ferromagnet. If the temperature of the material is below a critical value, it becomes ferromagnetic.
Conversely, to change the polarity of a ferromagnet, one usually needs to first heat it up above its critical temperature. The electron spins can then reorient themselves, and after cooling down, the magnetic field of the ferromagnet eventually points in a different direction.