Yes, you read that right. A 2025 study shows that gentle sound waves, not drugs, not gene editing, actually rolled back aging in cells and mice.


Edward Chang is a neurosurgeon, scientist, and a pioneering leader in functional neurosurgery and brain-computer interface technology, whose work spans the operating room, the research lab, and the engineering bench to restore speech and movement for patients who have lost these capabilities. In this episode, Edward explains the evolution of modern neurosurgery and its dramatic reduction in collateral damage, the experience of awake brain surgery, real-time mapping to protect critical functions, and the split-second decisions surgeons make. He also discusses breakthroughs in brain-computer interfaces and functional electrical stimulation systems, strategies for improving outcomes in glioblastoma, and his vision for slimmer, safer implants that could turn devastating conditions like ALS, spinal cord injury, and aggressive brain tumors into more manageable chronic illnesses.
View show notes here: https://bit.ly/46uJXlh.
Become a member to receive exclusive content: https://peterattiamd.com/subscribe/
Sign up to receive Peter’s email newsletter: https://peterattiamd.com/newsletter/
We discuss:
0:00:00 — Intro.
0:01:17 — The evolution of neurosurgery and the shift toward minimally invasive techniques.
0:10:58 — Glioblastomas: biology, current treatments, and emerging strategies to overcome its challenges.
0:17:39 — How brain mapping has advanced from preserving function during surgery to revealing how neurons encode language and cognition.
0:24:22 — How awake brain surgery is performed.
0:29:02 — How brain redundancy and plasticity allow some regions to be safely resected, the role of the corpus callosum in epilepsy surgery, and the clinical and philosophical implications of disconnecting the hemispheres.
0:43:46 — How neural engineering may restore lost functions in neurodegenerative disease, how thought mapping varies across individuals, and how sensory decline contributes to cognitive aging.
0:54:40 — Brain–computer interfaces explained: EEG vs. ECoG vs. single-cell electrodes and their trade-offs.
1:09:02 — Edward’s clinical trial using ECoG to restore speech to a stroke patient.
1:20:41 — How a stroke patient regained speech through brain–computer interfaces: training, AI decoding, and the path to scalable technology.
1:41:10 — Using brain-computer interfaces to restore breathing, movement, and broader function in ALS patients.
1:47:56 — The 2030 outlook for brain–computer interfaces.
1:52:35 — The potential of stem cell and cell-based therapies for regenerating lost brain function.
1:57:54 — Edward’s vision for how neurosurgery and treatments for glioblastoma, Parkinson’s disease, and Alzheimer’s disease may evolve by 2040
2:00:43 — The rare but dangerous risk of vertebral artery dissections from chiropractic neck adjustments and high-velocity movements.
2:02:31 — How Harvey Cushing might view modern neurosurgery, and how the field has shifted from damage avoidance to unlocking the brain’s functions.
——-
About:
The Peter Attia Drive is a deep-dive podcast focusing on maximizing longevity, and all that goes into that from physical to cognitive to emotional health. With over 90 million episodes downloaded, it features topics including exercise, nutritional biochemistry, cardiovascular disease, Alzheimer’s disease, cancer, mental health, and much more.
Peter Attia is the founder of Early Medical, a medical practice that applies the principles of Medicine 3.0 to patients with the goal of lengthening their lifespan and simultaneously improving their healthspan.
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/
What if the end of everything came not from cosmic fate, but from us? This episode examines the physics, probability, and peril of experiments that could, in theory, unravel the universe.
Watch my exclusive video The Economics of Immortality: https://nebula.tv/videos/isaacarthur–…
Get Nebula using my link for 40% off an annual subscription: https://go.nebula.tv/isaacarthur.
Get a Lifetime Membership to Nebula for only $300: https://go.nebula.tv/lifetime?ref=isa…
Use the link https://gift.nebula.tv/isaacarthur to give a year of Nebula to a friend for just $36.
Visit our Website: http://www.isaacarthur.net.
Join Nebula: https://go.nebula.tv/isaacarthur.
Support us on Patreon: / isaacarthur.
Support us on Subscribestar: https://www.subscribestar.com/isaac-a…
Facebook Group: / 1583992725237264
Reddit: / isaacarthur.
Twitter: / isaac_a_arthur on Twitter and RT our future content.
SFIA Discord Server: / discord.
Credits:
Could We Accidentally Destroy the Universe?
Written, Produced & Narrated by: Isaac Arthur.
Editors: Lukas Konecny.
Select imagery/video supplied by Getty Images.
Music Courtesy of Epidemic Sound http://epidemicsound.com/creator.
Chapters.
0:00 Intro.
2:38 Vacuum Decay (False Vacuum Collapse)
9:59 Strange Matter Conversion.
13:09 Gray Goo Scenario (Nanotechnology Out of Control)
16:05 Runaway Energy Reaction.
19:06 Altering the Constants of Nature.
20:49 Brane Collision (M-Theory Catastrophe)
22:27 Time Travel or Causality Paradox.
23:55 Nebula.
25:20 Simulation Shutdown.
27:21 Big Rip or Cosmological Instability.
28:35 Baby Universe Creation or Collapse.
29:51 Why It Hasn’t Happened Yet (Anthropic Principle & More)
31:49 Channel Updates
If I had the money this would be the first person I would call.
Can one forgotten organ hold the key to reversing aging? In this exclusive interview, Dr. Greg Fahy — one of the world’s leading longevity scientists — reveals groundbreaking discoveries about the thymus, age reversal, and the future of human health.
From regrowing his own thymus to pioneering cryobiology and organ preservation, Dr. Fahy shares insights that could change how we think about aging, immortality, and life extension. This conversation dives into the science behind reversing biological age, restoring the immune system, and even the possibility of medical time travel.
🔑 Topics covered in this video:
Thymus regeneration and why it may be the “master control” of aging.

A Harvard-affiliated study suggests that daily vitamin D supplementation may help slow biological aging by protecting DNA and preserving telomere length. The VITAL trial, which tracked over 1,000 adults for four years, found that participants taking 2,000 IU of vitamin D daily experienced less telomere shortening, effectively reducing biological aging by nearly three years.
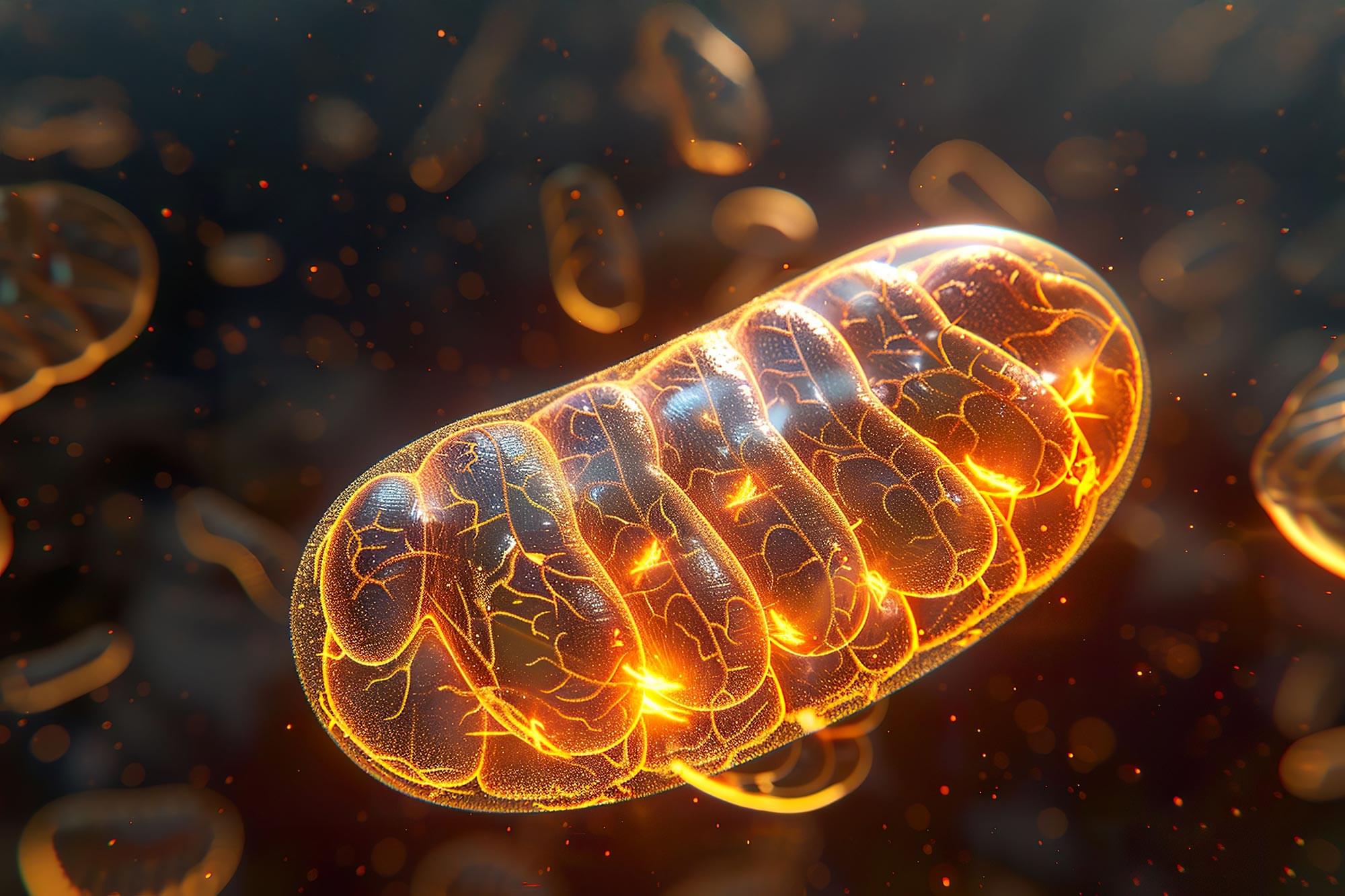
Sleep may serve as more than rest for the mind; it may also function as essential upkeep for the body’s energy systems. A new study from University of Oxford researchers, published in Nature, shows that the drive to sleep is caused by electrical stress building up in the tiny energy-producing structures of brain cells.
This finding provides a concrete physical explanation for the biological need for sleep and has the potential to reshape scientific thinking about sleep, aging, and neurological disorders.

A protein called ferritin light chain 1 (FTL1) may play a significant role in brain aging, a new study reveals, giving scientists a new target for understanding and potentially preventing brain deterioration and disease.
FTL1 was brought to light through a careful comparison of the hippocampus part of the brain in mice of different ages. The hippocampus is involved in memory and learning, and it is one of the regions that suffers most from age-related decline.
The study team found that FLT1 was the one protein in this region that old mice had more of and young mice had less of.