A new study charted how protein levels change along with human aging in many organs at once, comparing those shifts with RNA.


Dr. Esra Çavuşoğlu, PhD’s 71st Ayık Kafa podcast guest is Liz Parrish explores the transformative potential of gene therapy in extending human longevity and enhancing healthspan.
Liz Parrish MBA, is the Founder and CEO of BioViva Sciences USA Inc. BioViva is committed to extending healthy lifespans using gene therapy and works on combinatorial gene therapies with its proprietary CMV gene therapy delivery platform.
Liz is a humanitarian, entrepreneur, author, and innovator. In addition, she is a proponent of the Best Choice Medicine plan (BCM), a more efficient and streamlined regulatory model around the use of genetic therapies.
She shares her personal journey, the scientific basis for gene therapy, and the economic implications of aging-related diseases. The podcast also takes a closer look at the four different gene therapies that Liz Parrish administered to herself: Klotho, Follistatin, PGC-1α, and Telomerase Reverse Transcriptase. The discussion covers the various gene therapies being developed, their safety, ethical considerations, and the importance of early intervention. Liz emphasizes the need for public awareness and investment in longevity research to make these therapies accessible to all.
#EsraÇavuşoğlu #AyıkKafa #ElevatingLifeEvolvingHealth #Longevilab #LizParrish #Longevity #genetherapy.
Liz Parrish:

The human cerebral cortex is only a few millimetres thick and arranged in numerous folds. This tissue usually becomes thinner with age. “This is a hallmark of aging. It is attributed, among other things, to the loss of neurons. As a result, some abilities deteriorate. In any case, it is generally assumed that less brain volume means reduced function,” explains Prof. Esther Kühn, a neuroscientist at DZNE and the Hertie Institute for Clinical Brain Research. “However, little is known about how exactly the cortex actually ages. This is remarkable, given that many of our daily activities depend on a functioning cortex. That’s why we examined the situation with high-resolution brain scans.”
Together with colleagues from Tübingen and Magdeburg, Esther Kühn focused on a part of the cerebral cortex where signals from the tactile sense are processed. This “primary somatosensory cortex” is located on the left and right side of the top of the head and extends along a strip about a finger’s width wide towards each ear. “This brain area is relevant for the perception of one’s own body and for interacting with the environment,” explains the neuroscientist. “When I pick up a key, grasp a door handle or even walk, I constantly need haptic feedback to control my movements. The corresponding stimuli converge in this area and are also processed here”
Using magnetic resonance imaging (MRI), the researchers were able to map this area of the cerebral cortex with unprecedented accuracy. To do this, they employed a particularly sensitive scanner with a magnetic field strength of seven Tesla, enabling them to image minute brain structures about the size of a grain of sand. A total of around 60 women and men between the ages of 21 and 80 were examined. “Until now, it had not been considered that the primary somatosensory cortex consists of a stack of several extremely thin layers of tissue, each with its own architecture and function. We have now found that these layers age differently. Although the cerebral cortex becomes thinner overall, some of its layers remain stable or, surprisingly, are even thicker with age. Presumably because they are particularly solicited and thus retain their functionality. We therefore see evidence for neuroplasticity, that is, adaptability, even in senior people.”

In this Longevity Summit Dublin 2025 talk, Dr. David Furman (Buck Institute for Research on Aging) reveals how space medicine is becoming a powerful model for studying accelerated aging. From NASA collaborations to organoid experiments in simulated microgravity, Dr. Furman shows how heart, brain, and immune organoids age up to 10 years in just 24 hours — and how this can accelerate drug discovery for neurodegeneration, cardiovascular disease, and immune decline. Learn how microgravity research can predict your biological future and identify interventions to slow or reverse aging.
Chapters:
00:00 Introduction & NASA collaboration.
01:25 Accelerated aging in astronauts.
03:02 Simulating microgravity with organoids.
05:16 Brain, heart & immune system aging signatures.
07:03 Biological age clocks in organoids.
09:22 Parkinson’s, cardiomyopathy & immune dysfunction findings.
11:56 Translating microgravity science into longevity medicine.
13:43 Predicting future aging trajectories.
15:34 Beyond Age – a clinical test for aging projection.
16:17 Closing remarks.
#LongevityScience #AgingResearch #Microgravity #SpaceMedicine #BiologicalAge #LongevitySummit

Persistent genomic instability compromises cellular viability while also triggers non-cell-autonomous responses that drive dysfunction across tissues, contributing to aging. Recent evidence suggests that DNA damage activates secretory programs, including the release of inflammatory cytokines, damage-associated molecular patterns, and extracellular vesicles, that reshape immune homeostasis, stem cell function, and metabolic balance. Although these responses may initially support tissue integrity and organismal survival, their chronic activation has been associated with tissue degenerative changes and systemic decline. Here, we discuss how nuclear DNA damage responses trigger the activation of cytoplasmic sensing pathways, promote secretory phenotypes, and affect organismal physiology. Targeting DNA damage-driven mechanisms may help buffer harmful systemic responses while preserving regeneration and immune surveillance, offering new ways to delay aging-related decline.
© 2025 The Author(s). BioEssays published by Wiley‐VCH GmbH.
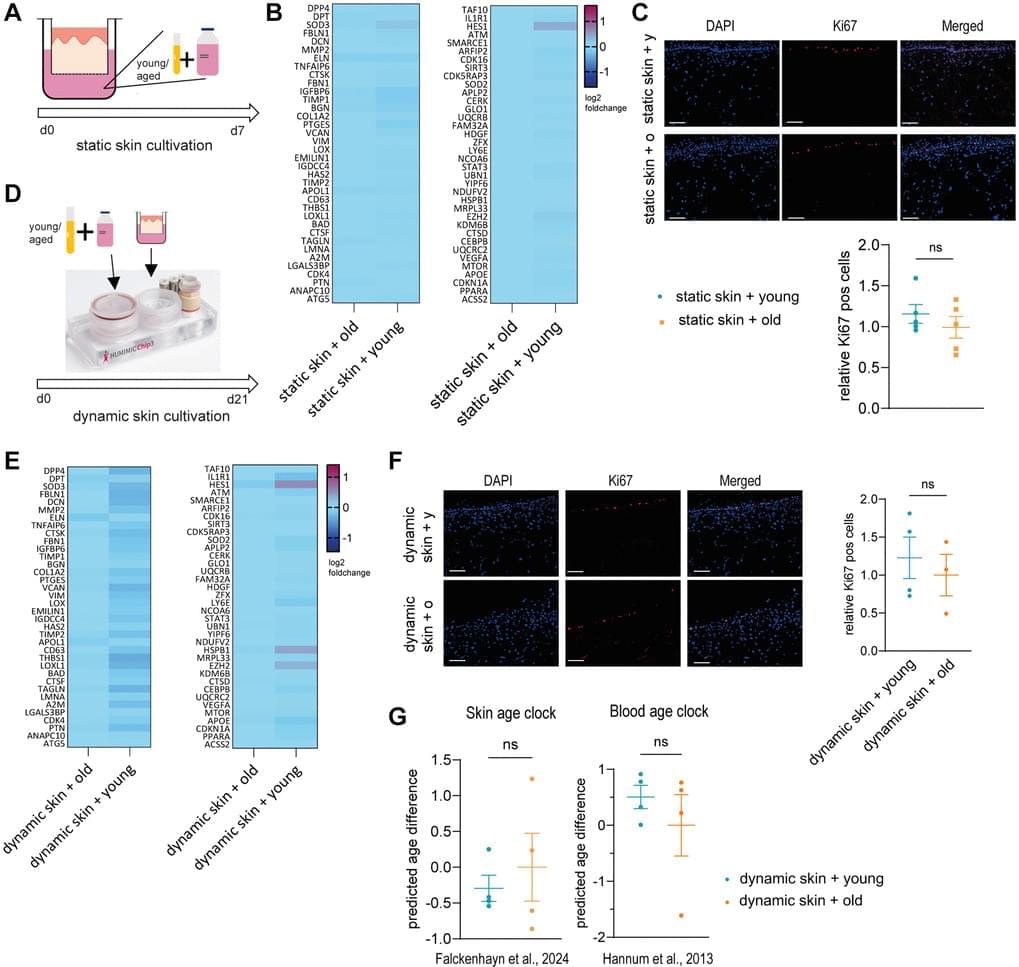
Aging is a complex process that significantly contributes to age-related diseases and poses significant challenges for effective interventions, with few holistic anti-aging approaches successfully reversing its signs. Heterochronic parabiosis studies illuminated the potential for rejuvenation through blood-borne factors, yet the specific drivers including underlying mechanisms remain largely unknown and until today insights have not been successfully translated to humans. In this study, we were able to recreate rejuvenation of the human skin via systemic factors using a microphysiological system including a 3D skin model and a 3D bone marrow model. Addition of young human serum in comparison to aged human serum resulted in an improvement of proliferation and a reduction of the biological age as measured by methylation-based age clocks in the skin tissue. Interestingly, this effect was only visible in the presence of bone marrow-derived cells. Further investigation of the bone marrow model revealed changes in the cell population in response to young versus aged human serum treatment. Using proteome analysis, we identified 55 potential systemic rejuvenating proteins produced by bone marrow-derived cells. For seven of these proteins, we were able to verify a rejuvenating effect on human skin cells using hallmarks of aging assays, supporting their role as systemic factors rejuvenating human skin tissue.
Aging | doi:10.18632/aging.206288. Johanna Ritter, Cassandra Falckenhayn, Minyue Qi, Leonie Gather, Daniel Gutjahr, Johannes Schmidt, Stefan Simm, Stefan Kalkhof, Janosch Hildebrand, Thomas Bosch, Marc Winnefeld, Elke Grönniger, Annette Siracusa.

For 25 years, scientists at Northwestern Medicine have been studying people aged 80 years and older – dubbed “SuperAgers” – to uncover what makes them stand out.
In a new study, researchers show that these individuals display memory performance comparable to those at least 30 years younger, defying the long-held belief that cognitive decline is an unavoidable part of aging.
The study was published in Alzheimer’s & Dementia.