Rapamycin makes animals live longer and boosts immunity in humans, but risks remain. Here is what this Easter Island longevity drug actually does.



The study unexpectedly identified a significant role for a group of enzymes known as agmatinases, which convert the metabolite agmatine into polyamines. These enzymes appear to participate in a previously unrecognized “metabolic feedback loop” that helps maintain balanced TOR activity. When agmatinase activity was disrupted, yeast cells grew more quickly but showed signs of premature aging, revealing a trade-off between rapid growth and long-term cell survival.
The team also found that adding agmatine or putrescine (a related compound) supported longevity in yeast and improved growth under specific conditions.
“By showing that agmatinases are essential for healthy aging, we’ve uncovered a new layer of metabolic control over TOR — one that may be conserved in humans,” said Dr. Rallis. “Because agmatine is produced by diet and gut microbes, this work may help explain how nutrition and the microbiome influence aging.”

“The long-term use of CBD is associated with less intense aggressive behaviors in dogs.”
Can cannabidiol (CBD) help dogs in the same way it helps humans? This is what a recent study published in Frontiers in Veterinary Science hopes to address as a team of scientists investigated the benefits of incorporating CBD products into dog products. This study has the potential to help scientists, legislators, and the public better understand the health benefits of CBD for both humans and animals.
For the study, the researchers analyzed data obtained from the Dog Aging Project (DAP), which is an organization designed to gain insight into dog aging, lifestyle, diet, and environmental factors. Surveys were conducted from 47,444 dog owners between December 2019 and December 2023, with the first surveys being s baseline regarding a dog’s overall health status, while the second survey was used to ascertain the amount of CBD or hemp the owners fed their dogs while also assessing changes in behavior and/or health.
In the end, the researchers found that healthy dogs were less likely to use CBD, whereas dogs with limiting health conditions like dementia, epilepsy, or cancer were more likely to use CBD. Additionally, CBD-use dogs were found to exhibit less aggressive behavior compared to non-use dogs. Finally, the team found that states where CBD was legal had higher rates of dogs using CBD.


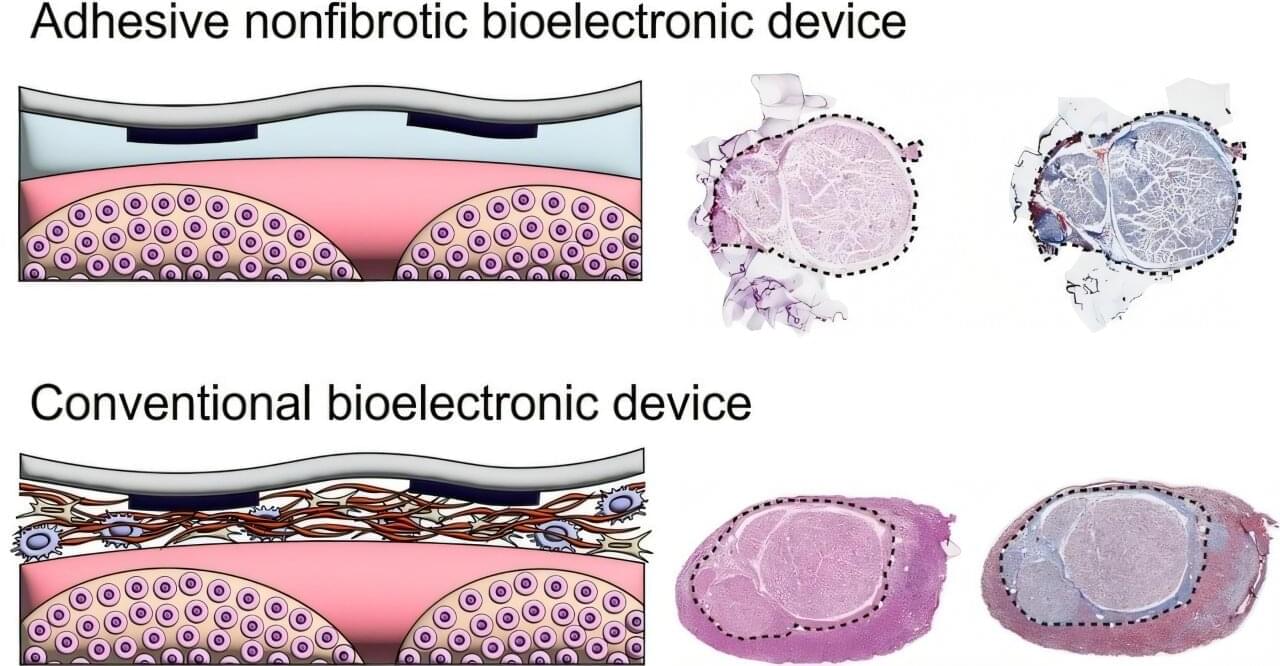
Peripheral nerves—the network connecting the brain, spinal cord, and central nervous system to the rest of the body—transmit sensory information, control muscle movements, and regulate automatic bodily functions. Bioelectronic devices implanted on these nerves offer remarkable potential for the treatment and rehabilitation of neurological and systemic diseases.
However, because the body perceives these implants as foreign objects, they often trigger the formation of dense fibrotic tissue at bioelectronic device–tissue interfaces, which can significantly compromise device performance and longevity.


