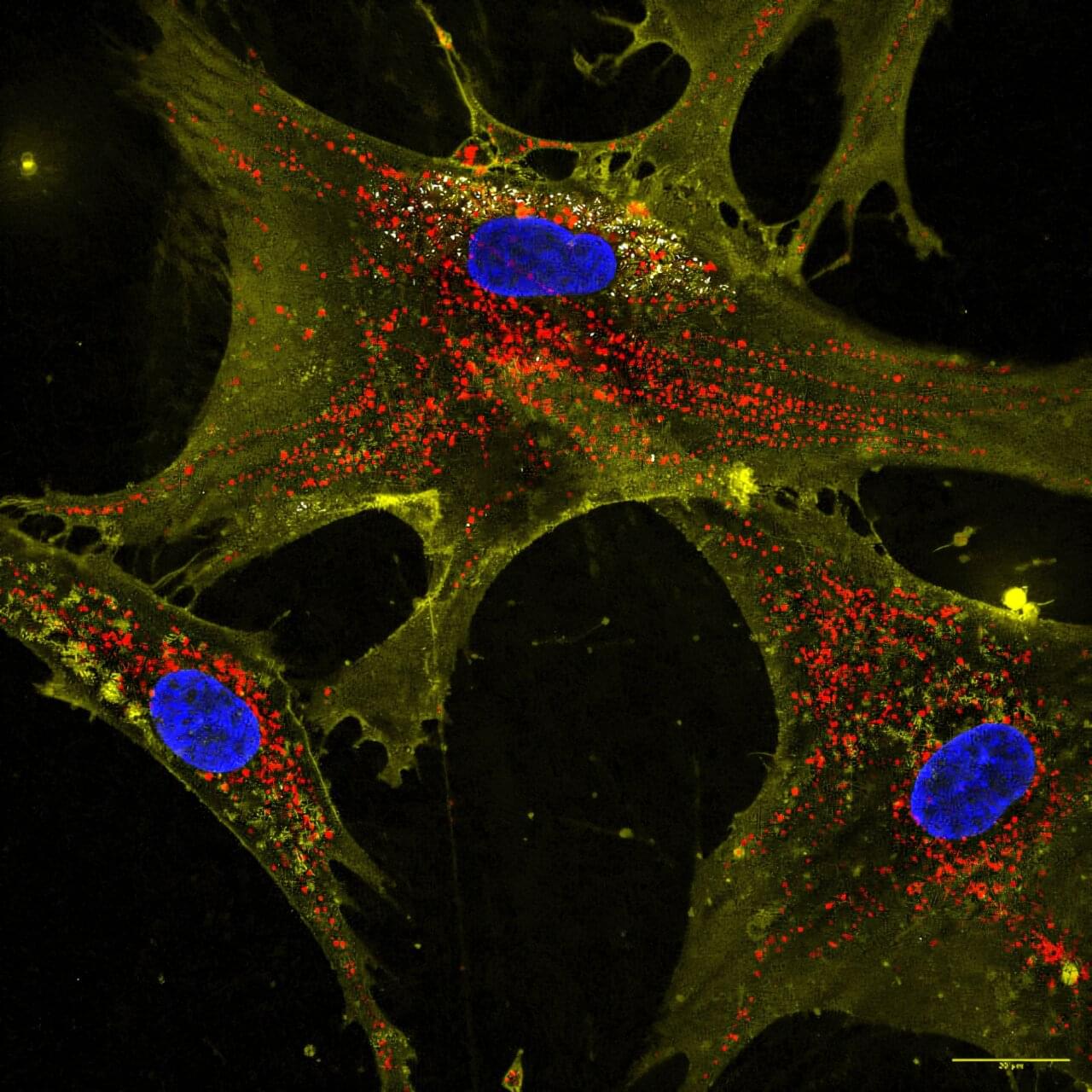
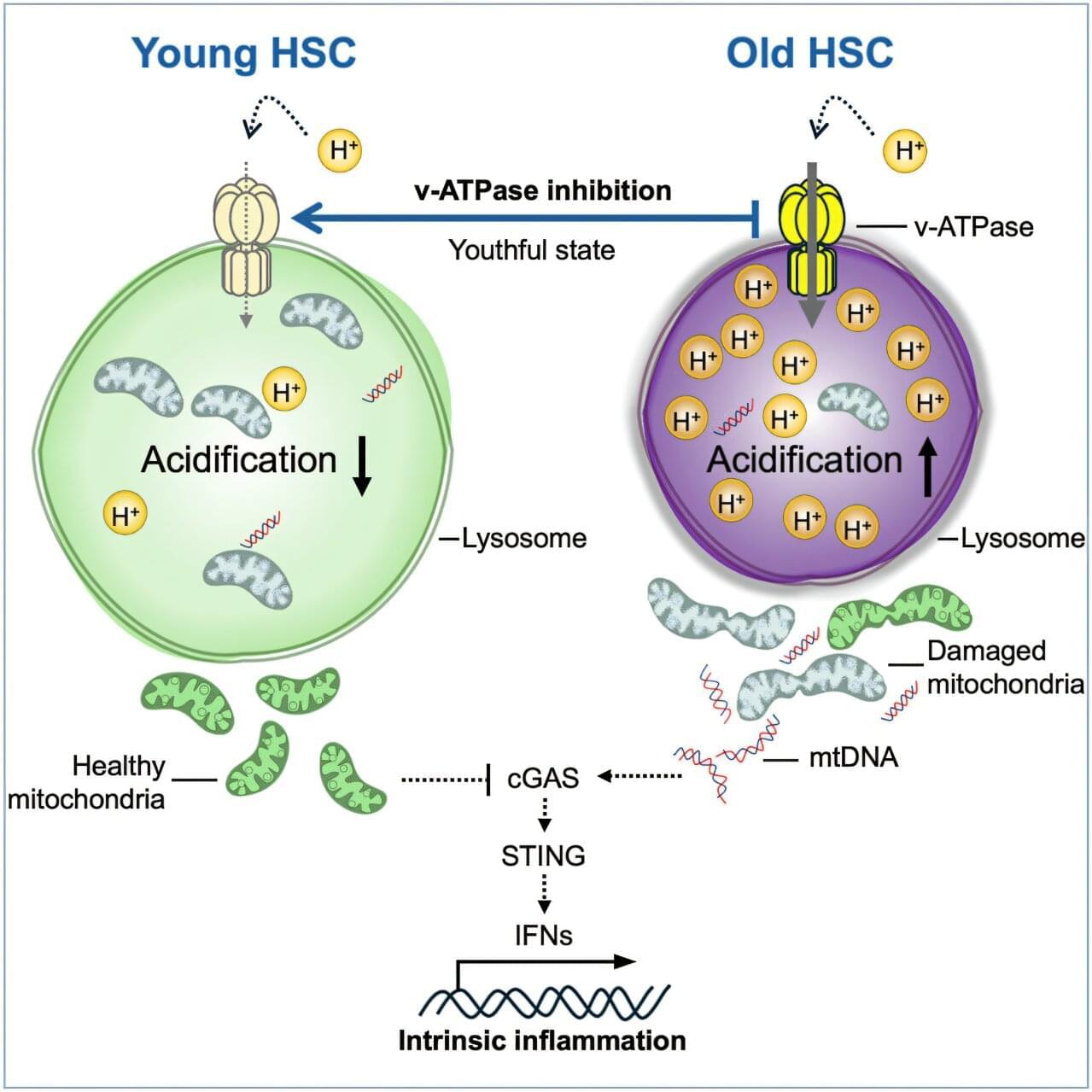
Researchers at the Icahn School of Medicine at Mount Sinai have discovered how to reverse aging in blood-forming stem cells in mice by correcting defects in the stem cell’s lysosomes. The breakthrough, published in Cell Stem Cell, identifies lysosomal hyperactivation and dysfunction as key drivers of stem cell aging and shows that restoring lysosomal slow degradation can revitalize aged stem cells and enhance their regenerative capacity.
Lysosomes are specialized structures that act as the cell’s recycling system, breaking down proteins, nucleic acids, carbohydrates, and lipids. Lysosomes accumulate and degrade waste, and eventually recycle it to be reused in biosynthetic processes. Lysosomes can also store nutrients to be released when needed. Lysosomes are recognized as pivotal for regulating metabolism in the cell, both catabolism (breaking down complex molecules to simple ones) and anabolism (building complex molecules from simpler ones).
The study, led by corresponding author Saghi Ghaffari, MD, Ph.D., Professor of Cell, Developmental, and Regenerative Biology at the Icahn School of Medicine and a member of the Black Family Stem Cell Institute, focuses on hematopoietic stem cells (HSCs). These are the rare long-lived cells in the bone marrow responsible for generating all blood and immune cells.