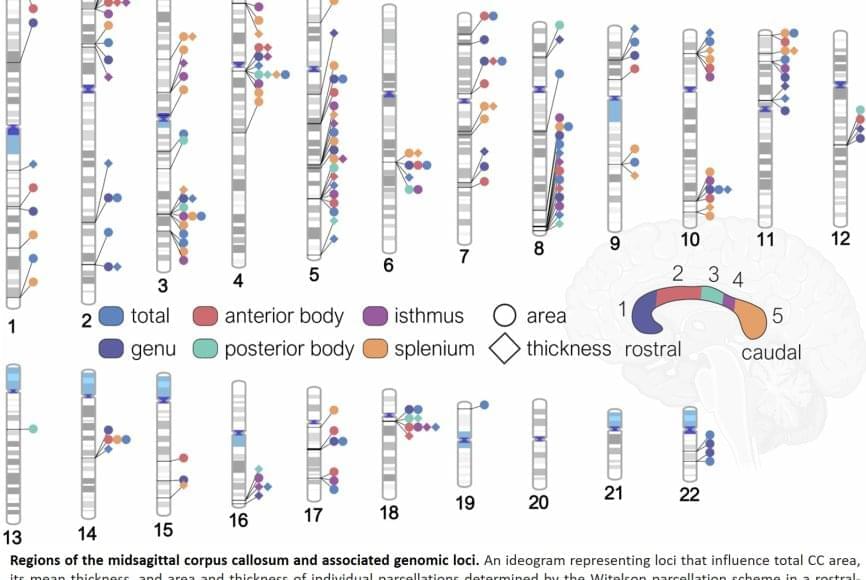
The corpus callosum is critical for nearly everything the brain does, from coordinating the movement of our limbs in sync to integrating sights and sounds, to higher-order thinking and decision-making. Abnormalities in its shape and size have long been linked to disorders such as ADHD, bipolar disorder, and Parkinson’s disease. Until now, the genetic underpinnings of this vital structure remained largely unknown.
In the new study, published in Nature Communications, the team analyzed brain scans and genetic data from over 50,000 people, ranging from childhood to late adulthood, with the help of a new tool the team created that leverages artificial intelligence.
“We developed an AI tool that finds the corpus callosum in different types of brain MRI scans and automatically takes its measurements,” said co-first author of the study. Using this tool, the researchers identified dozens of genetic regions that influence the size and thickness of the corpus callosum and its subregions.
The study revealed that different sets of genes govern the area versus the thickness of the corpus callosum—two features that change across the lifespan and play distinct roles in brain function. Several of the implicated genes are active during prenatal brain development, particularly in processes like cell growth, programmed cell death, and the wiring of nerve fibers across hemispheres.
Notably, the study found genetic overlap between the corpus callosum and the cerebral cortex—the outer layer of the brain responsible for memory, attention, and language—as well as with conditions such as ADHD and bipolar disorder.
For the first time, a research team has mapped the genetic architecture of a crucial part of the human brain known as the corpus callosum—the thick band of nerve fibers that connects the brain’s left and right hemispheres. The findings open new pathways for discoveries about mental illness, neurological disorders and other diseases related to defects in this part of the brain.