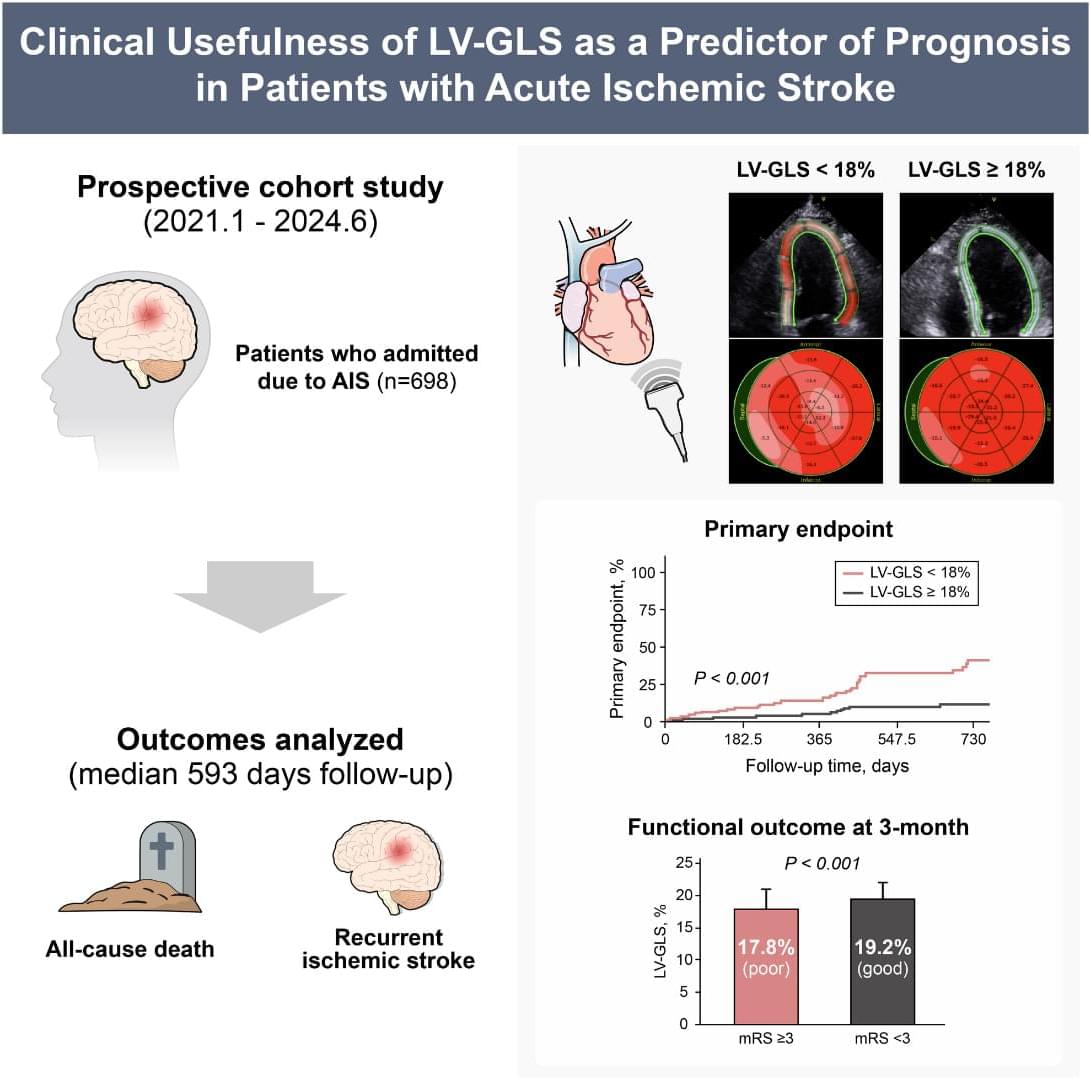
LV‐GLS 18% predicts mortality, recurrent stroke, and poor mRS-based functional outcome after acute ischemic stroke. @Minkwan_Kim84
LV‐GLS Globally, stroke is the second‐leading cause of death and the third most common cause of combined death and disability.1 Over the past decade, stroke‐related death has been steadily declining; however, health care expenditures associated with stroke have continued to increase.1, 2 Recurrence of ischemic stroke adversely affects patient prognosis and increases the mortality rate.3 Previous studies have identified several clinical factors contributing to the occurrence and recurrence of ischemic stroke, including stroke subtype, age, hypertension, atrial fibrillation (AF), heart failure (HF), and diabetes.2, 4
HF is also a risk factor for stroke and is associated with stroke recurrence and death.5, 6 Left ventricular (LV) global longitudinal strain (LV‐GLS), a measure of myocardial deformation along the long axis of the left ventricle, is assessed using the speckle‐tracking method. It is a sensitive measure of myocardial fiber shortening and has become a reliable parameter for evaluating subtle systolic dysfunction.7 In patients with acute HF, LV‐GLS is frequently reduced regardless of the LV ejection fraction (LVEF), the traditional measure of LV systolic function. LV‐GLS has also been shown to be a superior prognostic marker for death than LVEF.8 Furthermore, in severe mitral regurgitation and severe aortic stenosis, LV‐GLS has proven useful as a predictor of postoperative outcomes and a tool for identifying patients who may benefit from early surgical intervention.9, 10 Recent research has demonstrated that LV‐GLS can effectively predict incident strokes in patients who are stroke naïve.11 However, to date, no study has evaluated the prognostic implications of LV‐GLS in patients with acute ischemic stroke (AIS) about subsequent cardiovascular outcomes. In this study, we aimed to investigate the prognostic utility of LV‐GLS, a novel marker of subclinical LV dysfunction, in patients with AIS.