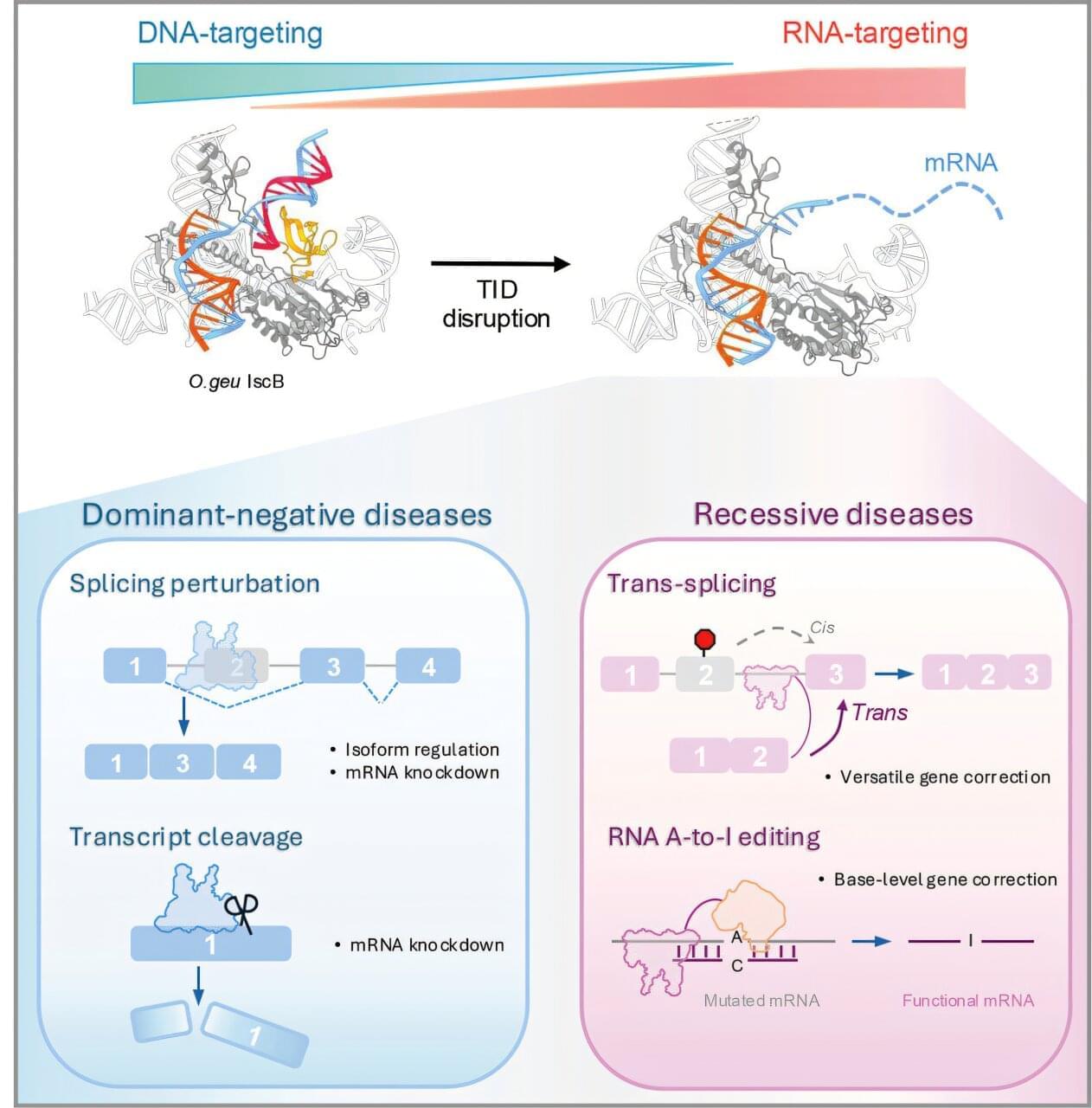
A new study has mapped the genetic blueprint of neural stem and progenitor cells (NPCs), the rare cells responsible for generating new neurons in the adult brain.

Chronic pain is life-changing and considered one of the leading causes of disability worldwide, making daily life difficult for millions of people around the world, and exacerbating personal and economic burdens. Despite established theories about the molecular mechanisms behind it, scientists have been unable to identify the specific processes in the body responsible, until now.
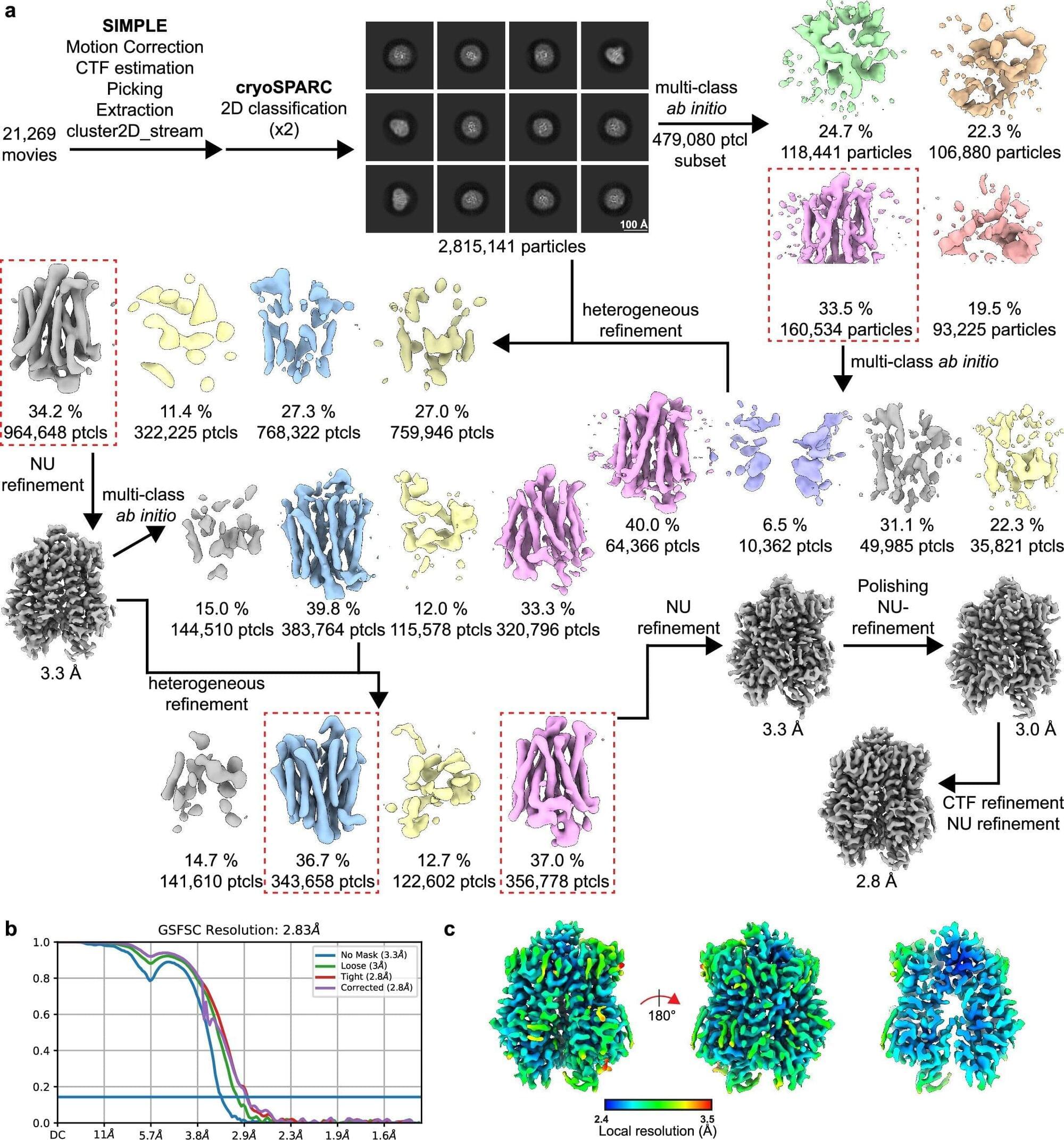
In an exciting collaboration, a team led by NDCN’s Professor David Bennett, and Professor Simon Newstead in the Department of Biochemistry and Kavli Institute for NanoScience Discovery, have identified a new genetic link to pain, determined the structure of the molecular transporter that this gene encodes, and linked its function to pain.
The findings of the research offers a promising, new, specific target against which to develop a drug to alleviate chronic pain. The paper “SLC45A4 is a pain gene encoding a neuronal polyamine transporter” is published in Nature.

A large international study led by researchers at the Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, shows that major depressive disorder (MDD) not only increases risk for a wide range of diseases and social problems, but is also partly driven by factors such as loneliness, obesity, smoking, and chronic pain.
The study, published in Nature Mental Health, applied genetic methods to systematically test which traits are causes, and which are consequences, of depression. The findings highlight the double burden of MDD: it both arises from and contributes to poor health, making prevention and treatment particularly urgent.
“We show that depression sits at the center of a web of health problems,” says Joëlle Pasman, research associate at Amsterdam UMC and Karolinska Institutet, who led the study. “It is not only a debilitating condition in itself but also increases the risk of many diseases, while at the same time being triggered by social, behavioral, and medical factors.”
Over the past 20 years, a class of cancer drugs called CD40 agonist antibodies have shown great promise—and induced great disappointment. While effective at activating the immune system to kill cancer cells in animal models, the drugs had limited impact on patients in clinical trials and caused dangerously systemic inflammatory responses, low platelet counts, and liver toxicity, among other adverse reactions—even at a low dose.
But in 2018, the lab of Rockefeller University’s Jeffrey V. Ravetch demonstrated it could engineer an enhanced CD40 agonist antibody so that it improved its efficacy and could be administered in a manner to limit serious side effects. The findings came from research on mice, genetically engineered to mimic the pathways relevant in humans. The next step was to have a clinical trial to see the drug’s impact on cancer patients.
Now the results from the phase 1 clinical trial of the drug, dubbed 2141-V11, have been published in Cancer Cell. Of 12 patients, six patients saw their tumors shrink, including two who saw them disappear completely.
The researchers demonstrate that an engineered antibody improves a class of drugs that has struggled to make good on its early promise.
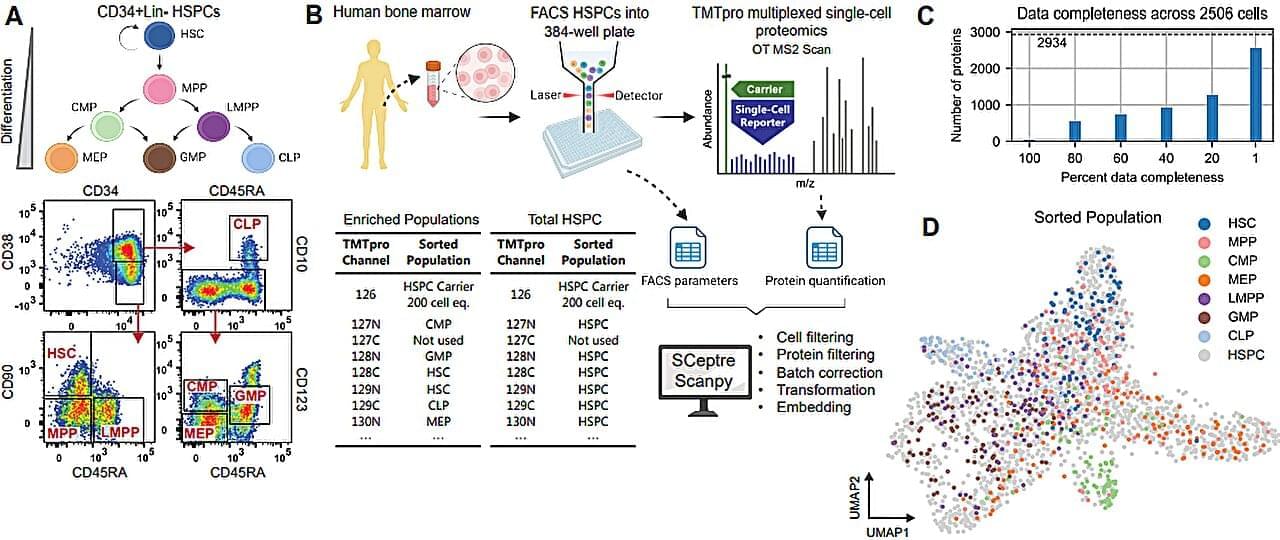
In the past decade, there has been significant interest in studying the expression of our genetic code down to the level of single cells, to identify the functions and activities of any cell through the course of health or disease.
The identity of a cell, and the way that identity can go awry, is critical to its role in many of the biggest health challenges we face, including cancer, neurodegeneration, or genetic and developmental disorders. Zooming in on single cells allows us to tell the difference between variants which would otherwise be lost in the average of a region. This is essential for finding new medical solutions to diseases.
Most single-cell gene expression experiments make use of a technology called single-cell RNA sequencing (scRNA-seq), which produces a map of exactly which genes are being copied out into short “transcripts” inside the nucleus. However, scRNA-seq only gives us a window into the intermediate step between the genetic code and the proteins which take care of (almost) all the tasks inside our bodies.
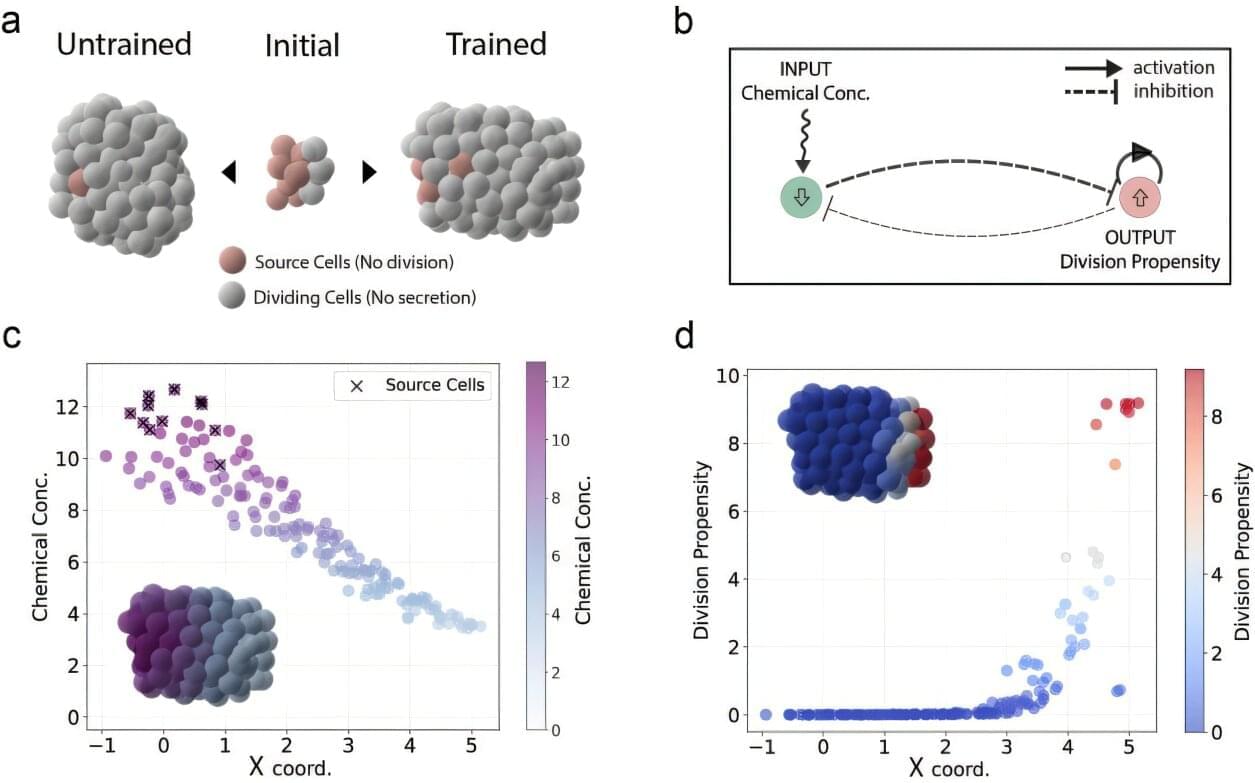
One of the most fundamental processes in all of biology is the spontaneous organization of cells into clusters that divide and eventually turn into shapes—be they organs, wings or limbs.
Scientists have long explored this enormously complex process to make artificial organs or understand cancer growth—but precisely engineering single cells to achieve a desired collective outcome is often a trial-and-error process.
Harvard applied physicists consider the control of cellular organization and morphogenesis to be an optimization problem that can be solved with powerful new machine learning tools. In new research published in Nature Computational Science, researchers in the John A. Paulson School of Engineering and Applied Sciences (SEAS) have created a computational framework that can extract the rules that cells need to follow as they grow, in order for a collective function to emerge from the whole.

An innovative method that uses modified versions of a bacterial virus effective at delivering treatments to human cells shows promise as a more inexpensive and efficient way to treat some deadly genetic diseases. Researchers from the School of Pharmacy at the University of Waterloo use a modified version of a bacterial virus called M13 to target specific human cells while
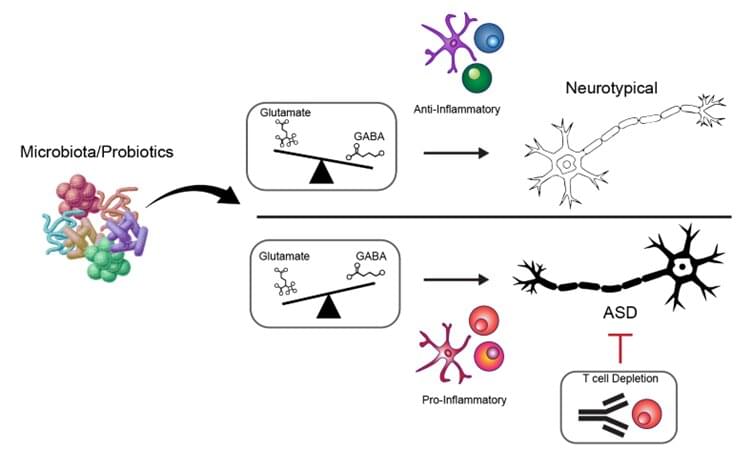
Autism spectrum disorder (ASD) affects an estimated 1 in 31 children in the United States by 2025, and prevalence in East Asian countries, such as South Korea, Singapore, and Japan, may be even higher than those in the United States. Despite its increasing prevalence, the underlying causes of ASD remain poorly understood, and there are currently no curative, preventive, or treatment options available.
A research team from POSTECH and ImmunoBiome in Korea, led by Professor Sin-Hyeog Im, who also serves as the CEO of ImmunoBiome, has made a discovery that reveals a multi-faceted mechanism behind ASD. This study, published in the July issue of Nature Communications, in collaboration with Dr. John C. Park and Prof. Tae-Kyung Kim, demonstrates that the gut microbiota and host immune system together can influence the progression of ASD in a genetic mouse model.
ASD has long been regarded as a genetically driven disorder. However, growing evidence suggests that environmental and microbial factors also play a role. The human gut harbors more than ten times as many microbial cells as human cells, and these microbes play vital roles in metabolism and the development of the immune system.