Genetic pet customization, designer pets 2050, futuristic pets, custom animal genetics, dna pet editing, crispr pets, designer dogs future, genetic engineering pets, bioengineered animals, create your own pet species, pet dna editing, designer cats 2050, genetic modified pets, custom pet labs, crispr cats, crispr dogs, futuristic pet ownership, biotechnology pets, glow in the dark pets, designer parrot genetics, genetic pet store future, engineered pets 2050, synthetic pets, build your own pet, dna modified animals, pet cloning future, futuristic pet breeds, genetic pets documentary, custom pets 2050, future animal technology, crispr pet design, genetically engineered pets, future dog breeds, custom parrot species, lab created pets, designer fish dna, genetic pet creation, future pet shops, crispr technology pets, bio pet labs future, create hybrid pets, animal dna editing, futuristic pet designs, design your own pet future, genetically customized animals, pet modification labs, genetic breeding future, synthetic biology pets, dna pet labs, engineered pet genetics, future hybrid pets, bioluminescent pets, pet dna science, designer turtles 2050, futuristic exotic pets, pet cloning labs, genetically programmed pets, dna modified species, designer hybrid animals, future of pets, biotechnology dogs, crispr engineered pets, pet dna modification, hybrid pets future, lab made pets, designer parrots, glowing pets dna, futuristic animal science, genetic modification animals, future pets prediction, dna engineered animals, custom pet species, crispr biotechnology pets, synthetic animal breeds, futuristic family pets, engineered exotic pets, dna edited dogs, glow in the dark cats, crispr futuristic pets, genetically designed pets, dna engineered cats, hybrid pet genetics, genetic evolution pets, pet dna design, engineered turtles, pet biotechnology future, futuristic pets science, genetic designer animals, crispr engineered dogs, dna pet programming, designer bio pets, dna hybrid animals, genetic dog breeds future, biotechnology cat design, future pet customization, dna edited parrots, bioengineering pets, custom pet dna science, crispr engineered animals, future designer pets 2050, dna reprogrammed animals, pet biotechnology industry, genetic engineered parrots, engineered hybrid dogs, futuristic pet labs, dna custom pets, genetic engineered cats, crispr glowing pets, biotechnology future pets, genetic science pets, future pet dna editing, lab created exotic pets, dna modified exotic animals, engineered pet cloning, futuristic pet market, genetic engineered hybrid pets, future animal dna science, biotechnology glowing pets, synthetic dna animals, futuristic pet lovers, pet dna labs 2050, engineered pet stores, custom dna pets future, future glowing cats, crispr glowing dogs, bioengineered hybrid pets, designer dna parrots, pet dna bio labs, futuristic exotic pet design, dna animal modification, engineered mini elephants, genetic designed turtles, futuristic pets 2070, dna modification in pets, custom engineered pets, biotechnology hybrid species, genetic glowing animals, pet dna engineering labs, designer pet hybrids, dna created exotic pets, future pet technology, genetically edited pets, bio custom pet stores, engineered pet dna future, dna glowing parrots, designer genetic cats, future biotechnology pets, crispr custom pets, engineered family pets, dna hybrid parrots, future pet science documentary, pet dna labs future, engineered dog species, future bioengineered animals, pet cloning 2050, custom glowing pets, biotechnology engineered cats, crispr glowing animals, genetically designed parrots, futuristic pet lab science, dna engineered turtles, designer animal dna, engineered futuristic pets, pet biotechnology predictions, dna designer pets, genetic engineered glowing animals, future pet design 2050, dna bioengineered cats, futuristic hybrid pet market, crispr engineered exotic pets, engineered glowing dogs, dna glowing exotic pets, biotechnology pet predictions, futuristic designer pets science, engineered pet bio labs, dna exotic animal design, genetic glowing turtles, future engineered animal species, custom dna exotic pets, crispr designed parrots, engineered glowing cats, futuristic pet dna bio labs, biotechnology glowing dogs, dna engineered glowing parrots, engineered pet biotechnology labs, custom dna glowing pets, future engineered exotic animals, genetic designed dogs, crispr engineered glowing cats, future biotechnology exotic pets, engineered hybrid parrots, dna glowing turtles, engineered exotic pet labs, genetic bio engineered animals, futuristic dna pet predictions.
Category: genetics – Page 55
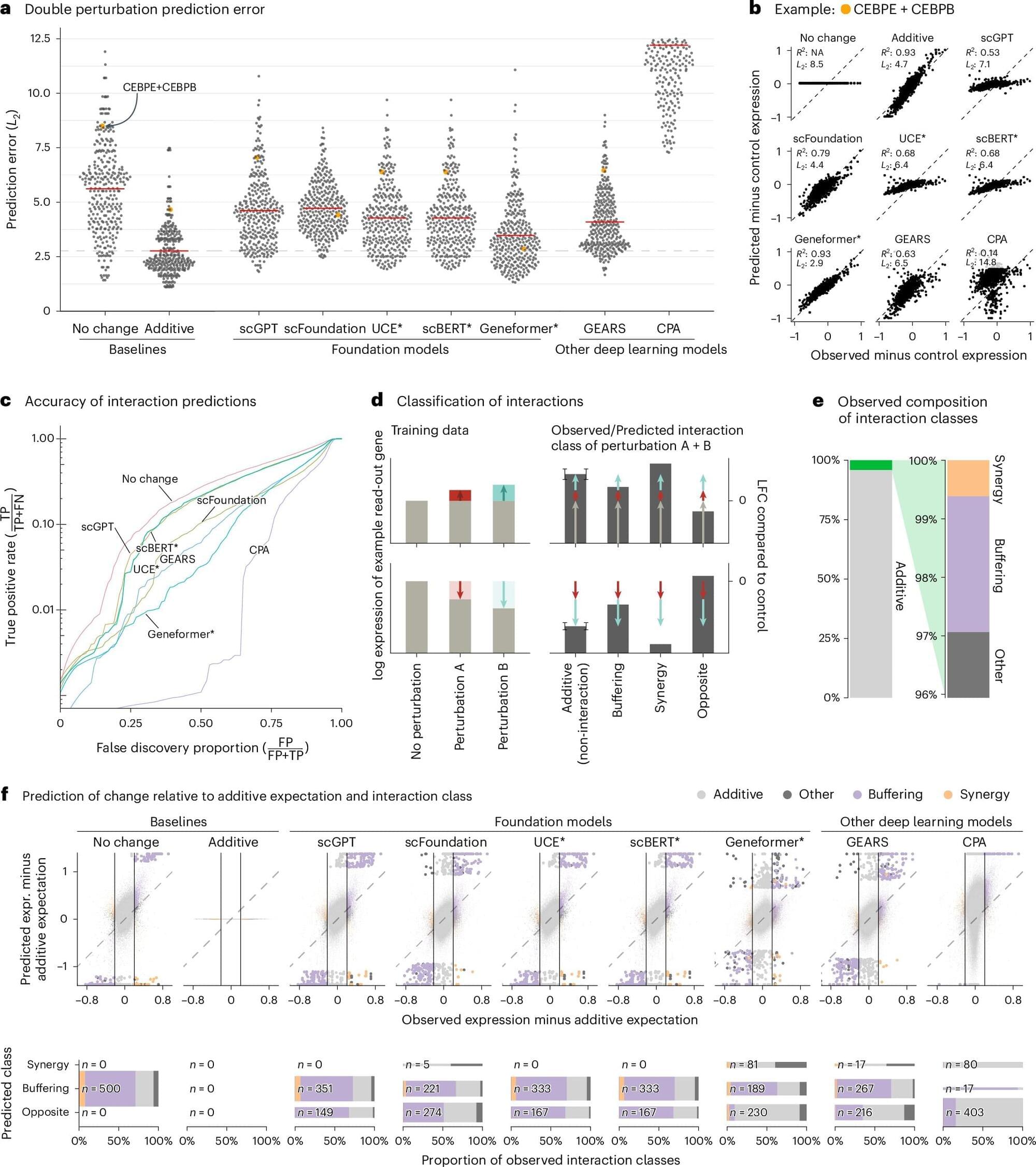


Genomics pioneer George Church earns first retraction for anti-aging gene therapy paper
A paper coauthored by geneticist George Church has been retracted following an internal review at a university where several coauthors are based.
The article appeared in the Proceedings of the National Academy of Sciences in 2022. The work supports an anti-aging gene therapy developed by BioViva, a company for which Church serves as an adviser. The paper’s authors claim cytomegalovirus (CMV) can be a gene therapy vector for a treatment for “aging-associated decline” that can be inhaled or injected monthly.
The work has been cited 41 times, two of which are citations from corrections to the article, according to Clarivate’s Web of Science.

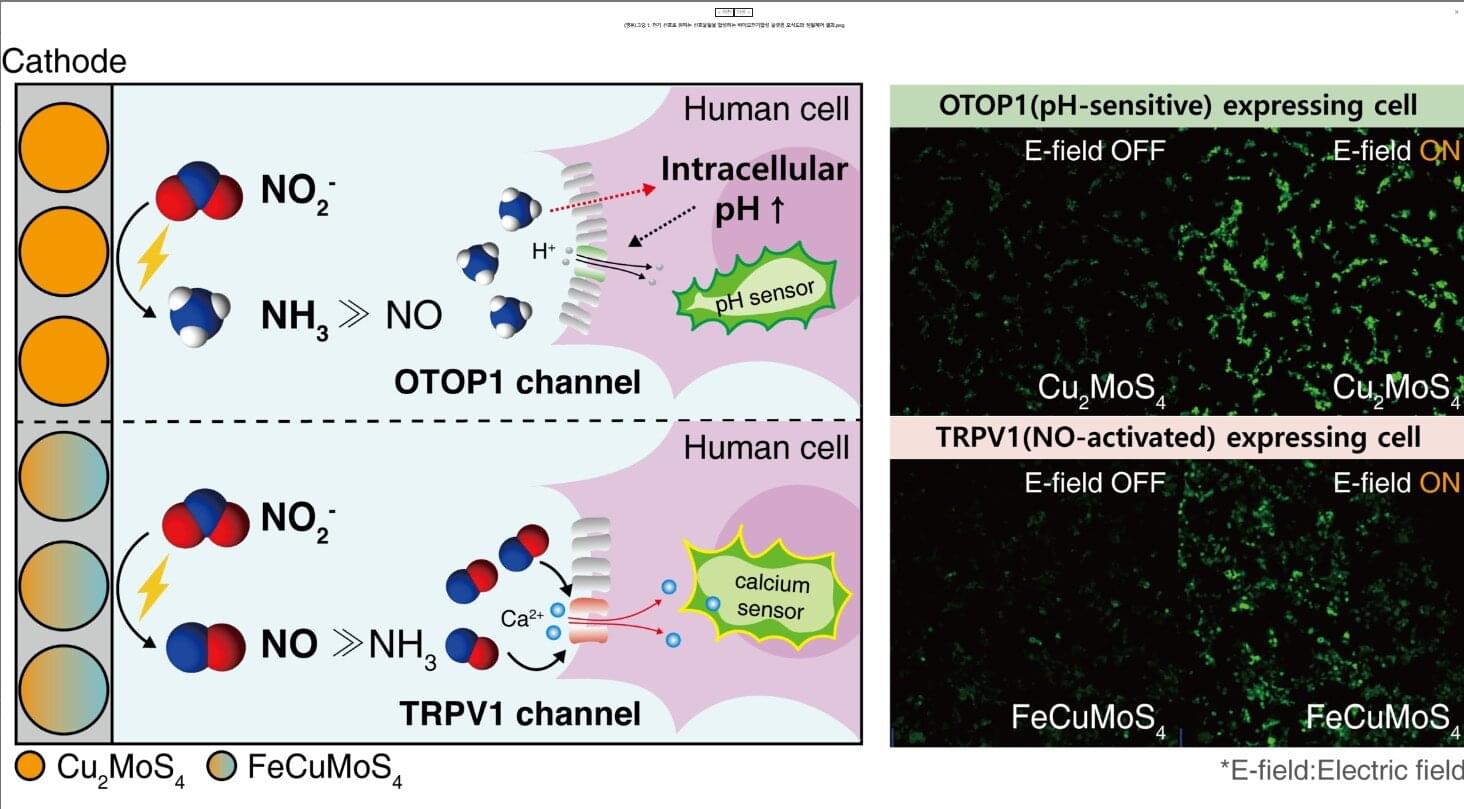
Bioelectrosynthesis platform enables switch-like, precision control of cell signaling
Cells use various signaling molecules to regulate the nervous, immune, and vascular systems. Among these, nitric oxide (NO) and ammonia (NH₃) play important roles, but their chemical instability and gaseous nature make them difficult to generate or control externally.
A KAIST research team has developed a platform that generates specific signaling molecules in situ from a single precursor under an applied electrical signal, enabling switch-like, precise spatiotemporal control of cellular responses. This approach could provide a foundation for future medical technologies such as electroceuticals, electrogenetics, and personalized cell therapies.
The research team led by Professor Jimin Park from the Department of Chemical and Biomolecular Engineering, in collaboration with Professor Jihan Kim’s group, has developed a bioelectrosynthesis platform capable of producing either nitric oxide or ammonia on demand using only an electrical signal. The platform allows control over the timing, spatial range, and duration of cell responses.
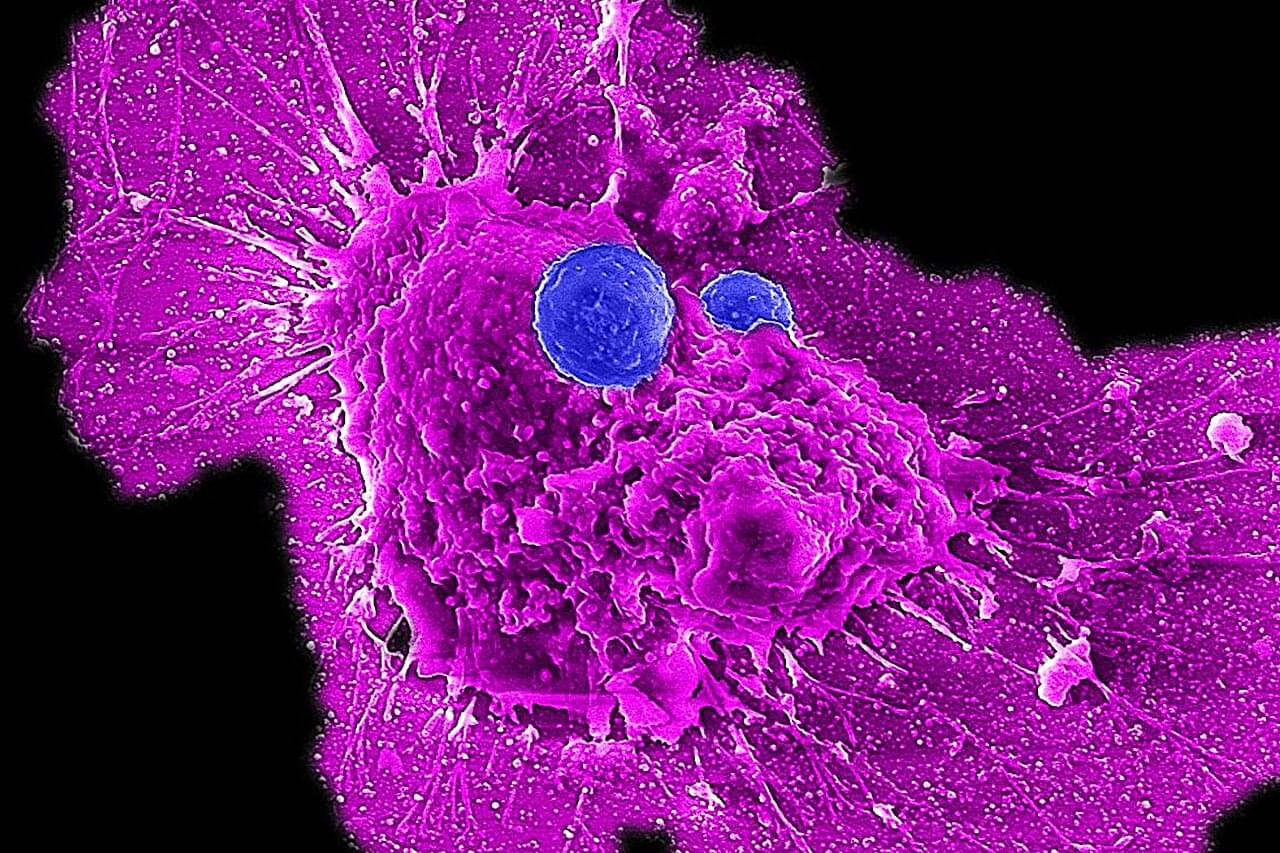
Scientists develop off-the-shelf immunotherapy for ovarian cancer
Ovarian cancer is the leading cause of death among women with gynecological cancers. The current medical playbook—surgery followed by chemotherapy—initially shows promise. Tumors shrink, sometimes disappearing entirely. But in more than 80% of patients, the cancer not only comes back, but returns more aggressive and increasingly resistant to the very treatments that once seemed effective.
But now, there could be new hope. In a study published in the journal Med, UCLA researchers have detailed their development of a new type of immune cell therapy, called CAR-NKT cell therapy, that could transform ovarian cancer care by delivering superior cancer-fighting power.
“This is the culmination of over a decade of work in my lab and represents over six years of collaboration with gynecologic oncologist Dr. Sanaz Memarzadeh,” said co-senior author Lili Yang, a professor of microbiology, immunology and molecular genetics and a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA.

New Study Suggests Cancer Drug Could Be Used to Target Protein Connection That Spurs Parkinson’s Disease
In studies with genetically engineered mice, Johns Hopkins Medicine researchers say they have identified a potentially new biological target involving Aplp1, a cell surface protein that drives the spread of Parkinson’s disease-causing alpha-synuclein.
The findings, published May 31 2024 in Nature Communications, reveal how Aplp1 connects with Lag3, another cell surface receptor, in a key part of a process that helps spread harmful alpha-synuclein proteins to brain cells. Those protein buildups are hallmarks of Parkinson’s disease.
Notably, the researchers say, Lag3 is already the target of a combination cancer drug approved by the U.S. Food and Drug Administration (FDA) that uses antibodies to “teach” the human immune system what to seek and destroy.