A new study shows that gene therapy can significantly improve hearing in both children and adults with congenital deafness caused by mutations in the OTOF gene.

I believe that dna will be able to answer just about all our genetic coding questions so much that it will lead to even better breakthroughs in the future and use hardly any energy. I believe also that the master algorithm can eventually be derived from DNA as dna seems already a perfect master algorithm for human beings where human beings are the key to all future progress. I say this as quantum computing is still not stable but we already know that dna computers seem already a masterpiece already especially even organoids of the human brain. Really it becomes really quite simple as even the quantum realm is unstable but dna computers that are quantum would stabilize this currently unstable realm.
Riera Aroche, R., Ortiz García, Y.M., Martínez Arellano, M.A. et al. DNA as a perfect quantum computer based on the quantum physics principles. Sci Rep 14, 11,636 (2024). https://doi.org/10.1038/s41598-024-62539-5
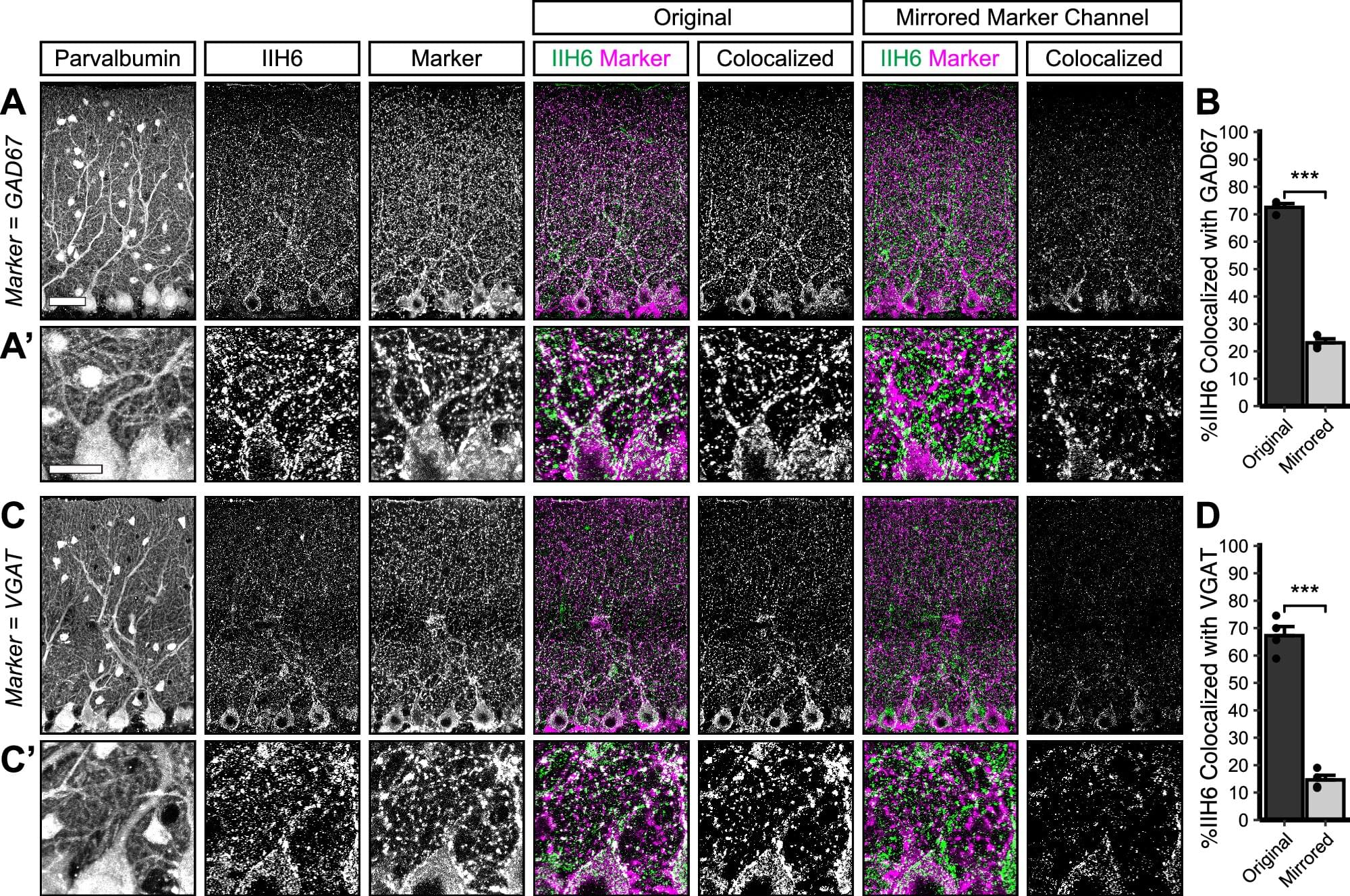
Scientists have uncovered how a protein helps build and maintain vital brain connections, providing insights into the neurological problems experienced by people with a rare form of muscular dystrophy known as dystroglycanopathy.
The research conducted at Oregon Health & Science University and published in Communications Biology reveals that the protein Dystroglycan plays a critical role in forming and maintaining connections between nerve cells in the cerebellum—the part of the brain responsible for movement coordination and motor learning.
In people with dystroglycanopathy, genetic mutations in the protein affect not only muscles but also the brain. The condition is a type of congenital muscular dystrophy, a group of inherited disorders that appear at birth or in early infancy.

A study published in Cell Stem Cell reveals that some mutations in blood stem cells might help protect against late-onset Alzheimer’s disease.
A team led by researchers at Baylor College of Medicine discovered that both a mouse model and people carrying blood stem cells with mutations in the gene TET2, but not in the gene DNMT3A, had a lower risk of developing Alzheimer’s disease. Their study proposes a mechanism that can protect against the disease and opens new avenues for potential strategies to control the emergence and progression of this devastating condition.
“Our lab has long been studying blood stem cells, also called hematopoietic stem cells,” said lead author Dr. Katherine King, professor of pediatrics— infectious diseases and a member of the Center for Cell and Gene Therapy and the Dan L Duncan Comprehensive Cancer Center at Baylor. She is also part of Texas Children’s Hospital.
Scientists from QIMR Berghofer’s Cardiac Bioengineering Lab have developed lab-grown, three-dimensional heart tissues known as cardiac organoids that mimic the structure and function of real adult human heart muscle.
To create these tissues, the researchers use special cells called human pluripotent stem cells (which can turn into any cell in the body). However, when these stem cells become heart cells, they usually stay immature and more like the heart tissue found in a developing baby. This immaturity can limit their usefulness to model diseases that present in childhood or as an adult.
In the study, researchers activated two key biological pathways to mimic the effects of exercise in order to mature these cells, making them behave more like genuine adult heart tissue. This breakthrough means scientists can now use these lab-grown heart tissues to test new drugs that could help people with heart conditions. The findings have been published in Nature Cardiovascular Research.
New cutting-edge software developed in Melbourne can help uncover how the most common heart tumor in children forms and changes. And the technology has the potential to further our understanding of other childhood diseases, according to a new study.
The research, led by Murdoch Children’s Research Institute (MCRI) and published in Genome Biology, found the software, VR-Omics, can identify previously undetected cell activities of cardiac rhabdomyoma, a type of benign heart tumor.
Developed by MCRI’s Professor Mirana Ramialison, VR-Omics is the first tool capable of analyzing and visualizing data in both 2D and 3D virtual reality environments. The innovative technology aims to analyze the spatial genetic makeup of human tissue to better understand a specific disease.
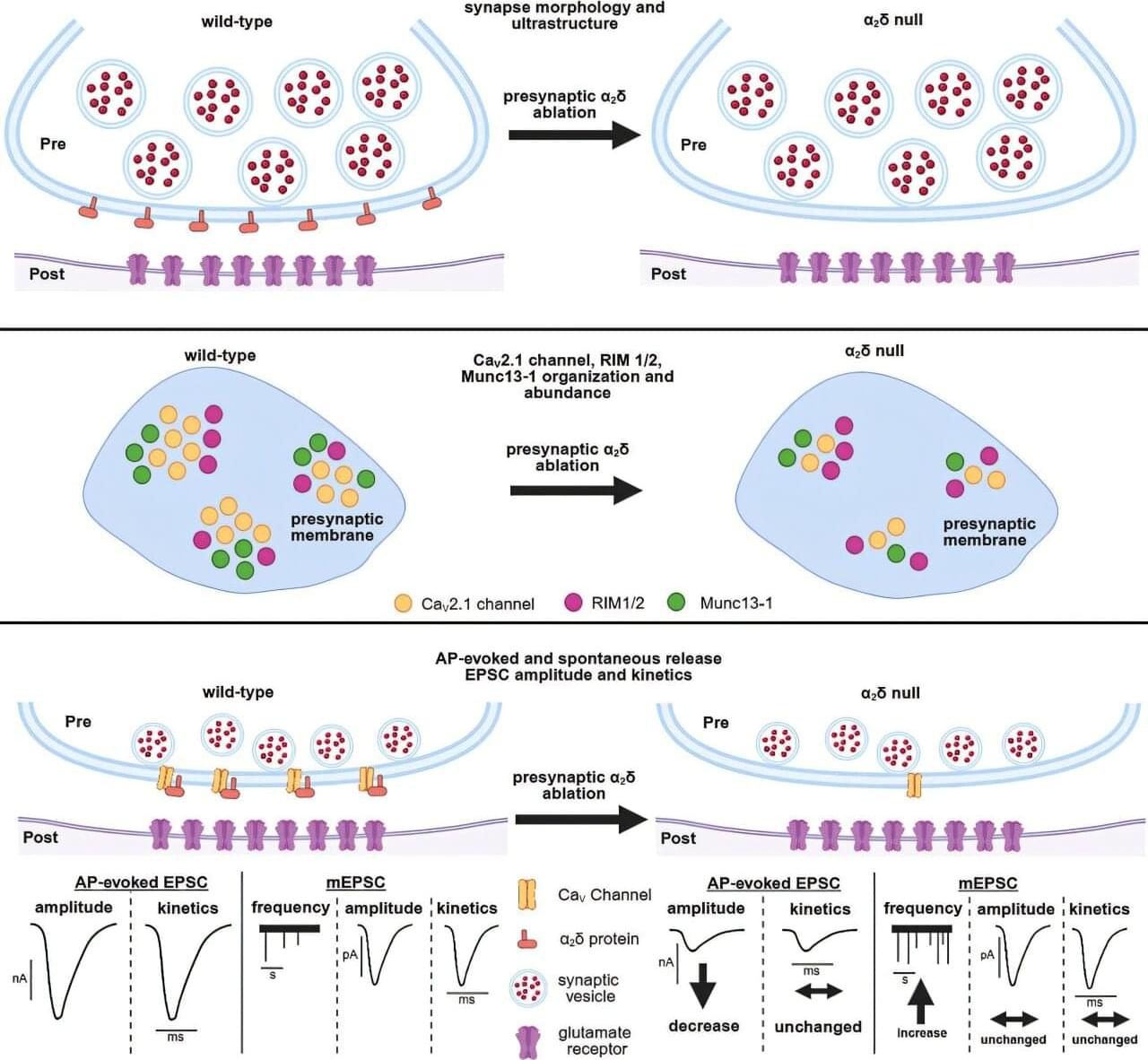
Cellular communication between neurons within our brain is complex and busy, much like a USPS mailroom.
To keep things running smoothly, the brain uses specialized molecules, termed alpha-2-delta (α2δ) proteins, to coordinate the sending and receival of signals between nerve cells in the brain.
Genetic variations in these types of proteins can impact important brain messaging and function, resulting in chronic pain, autism spectrum disorders, epilepsy, migraines, and other conditions.

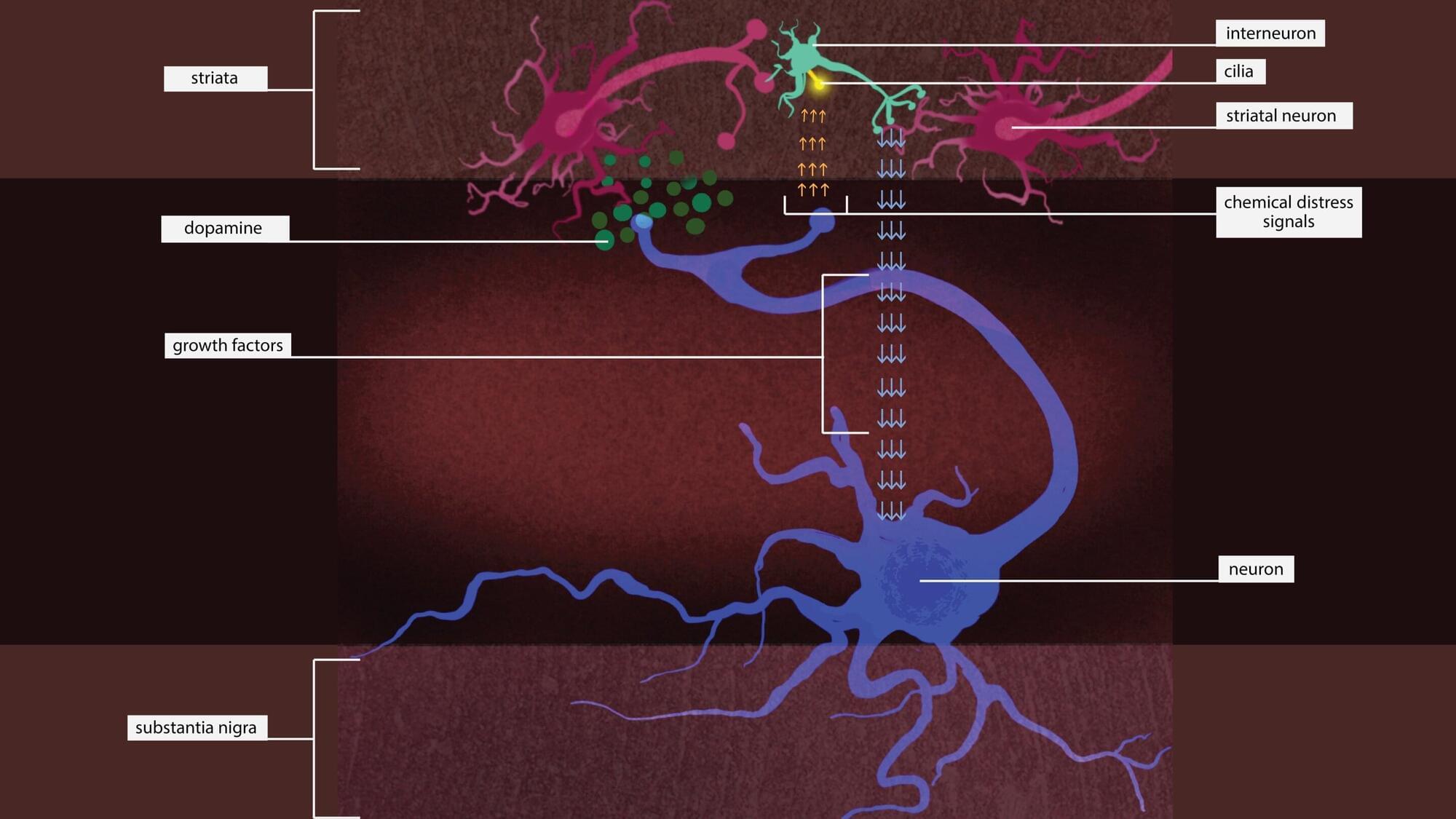
Putting the brakes on an enzyme might rescue neurons that are dying due to a type of Parkinson’s disease that’s caused by a single genetic mutation, according to a new Stanford Medicine-led study conducted in mice.
The study has been published in Science Signaling.
The genetic mutation causes an enzyme called leucine-rich repeat kinase 2, or LRRK2, to be overactive. Too much LRRK2 enzyme activity changes the structure of brain cells in a way that disrupts crucial communication between neurons that make the neurotransmitter dopamine and cells in the striatum, a region deep in the brain that is part of the dopamine system and is involved in movement, motivation and decision-making.
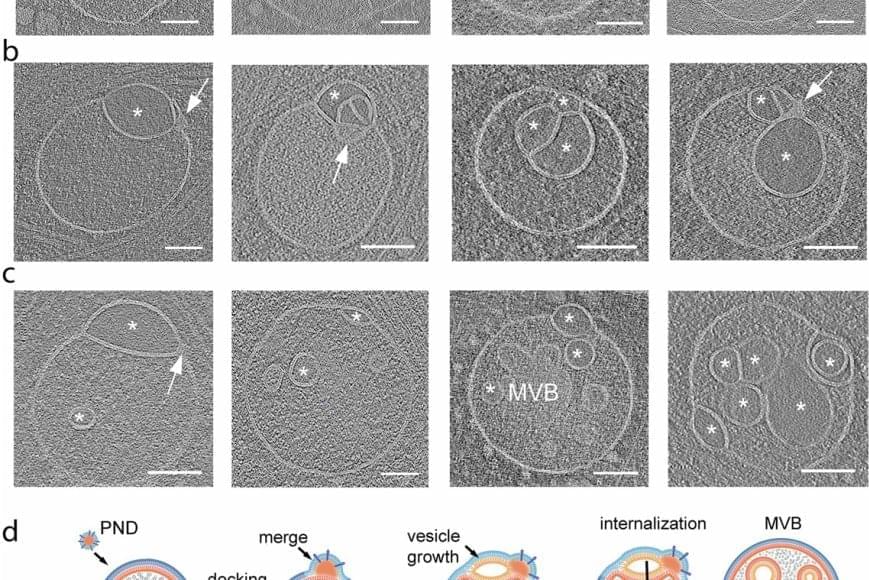
The discovery of an unknown organelle inside our cells could open the door to new treatments for devastating inherited diseases.
The organelle, a type of specialized structure, has been dubbed a “hemifusome” by its discoverers. This little organelle has a big job helping our cells sort, recycle and discard important cargo within themselves, the scientists say. The new discovery could help scientists better understand what goes wrong in genetic conditions that disrupt these essential housekeeping functions.
One such condition is Hermansky-Pudlak syndrome, a rare genetic disorder that can cause albinism, vision problems, lung disease and issues with blood clotting. Problems with how cells handle cargo are at the root of many such disorders.
The scientists believe hemifusomes facilitate the formation of vesicles, tiny blister-like sacs that act as mixing bowls, and of organelles made up of multiple vesicles. This process is critical to cellular sorting, recycling and debris disposal, the researchers report.