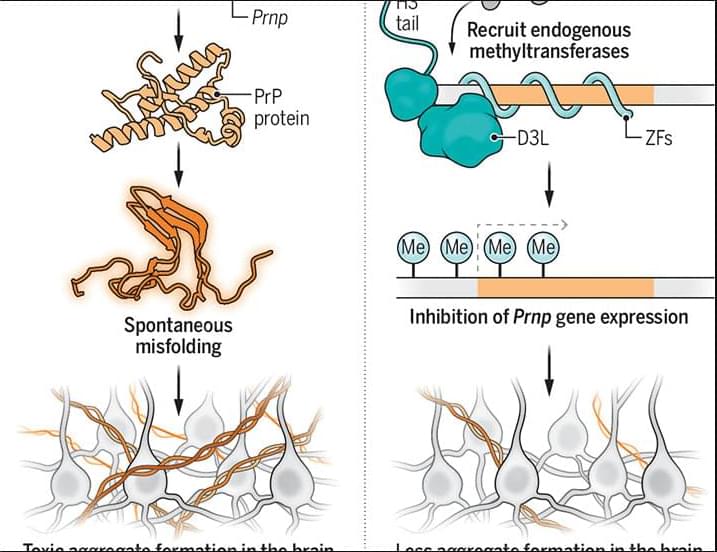
Not all genes respond in the same way to regulation by the same molecule—a property that might enable cells to produce complex genetic responses.
Genes in living cells may become active or may be suppressed in response to environmental stimuli such as heat or the availability of nutrients. For bacteria, this gene regulation often appears to be a simple “on-off switch” controlled by regulatory proteins called transcription factors (TFs). But researchers have now found that different genes might respond differently to the same stimulus even if they are regulated by the same TF [1]. The team activated genes involved in DNA repair and observed gene-to-gene variations in their protein production patterns. Such differences might have been exploited by evolution to achieve complex responses with relatively few molecular components, the researchers suggest.
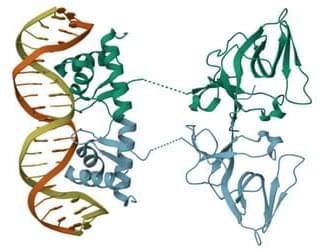
In the typical scenario, a TF binds to a region of a so-called promoter, a DNA sequence next to a gene. If the TF is the type that blocks gene expression, it prevents the enzyme RNA polymerase from binding and thus from beginning the process of producing the protein that the gene encodes. Because of thermal fluctuations (noise), the TF may spontaneously unbind, allowing gene expression to proceed until it rebinds. The rate of TF binding depends on its concentration, so fluctuations in concentration will cause changes in gene expression.