A new study shows that genetic evidence of historical contact between populations reveals consistent patterns of language change.
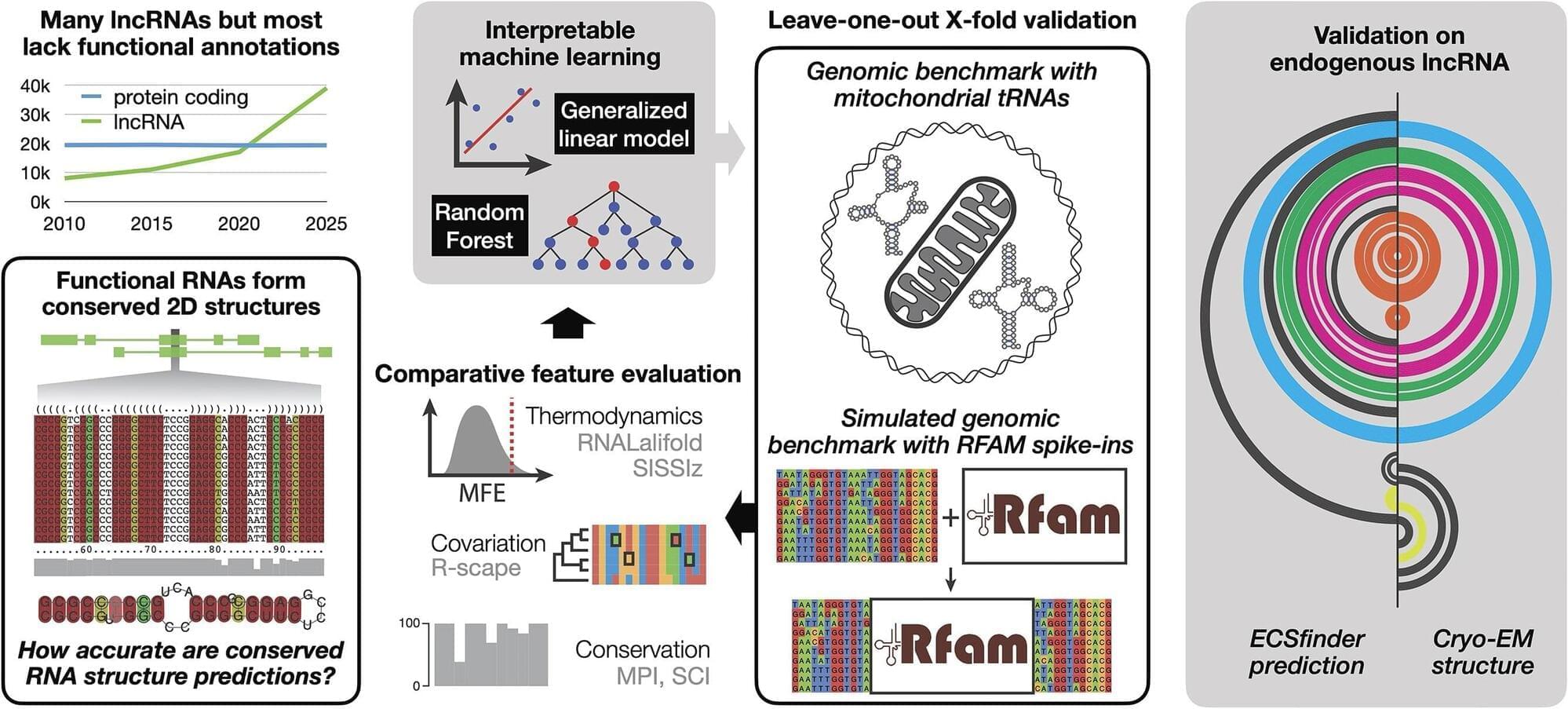
We mapped the human genome decades ago, but most of it is still a black box. Now, UNSW scientists have developed a tool to peer inside and what they find could reshape how we think about disease.
Your genome is the genetic map of you, and we understand almost none of it.
Our handle on the bits of the genome that tell the body how to do things (“make eyes blue,” “build heart tissue,” “give this person sickle cell anemia”) is OK, but there are vast areas of the genome that don’t appear to do anything.
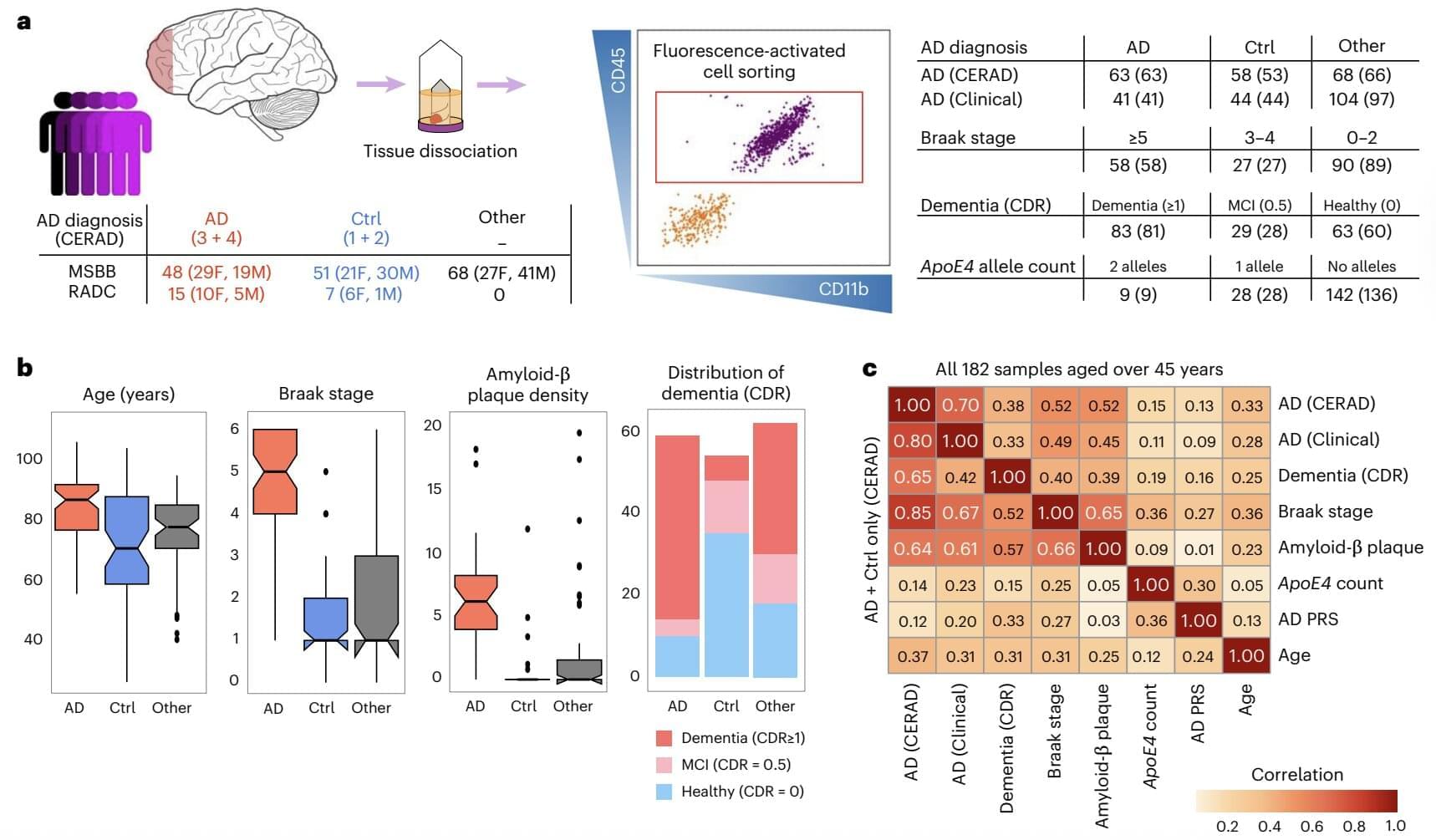
Alzheimer’s disease (AD) is a debilitating neurodegenerative disorder that causes progressive memory loss and a decline in mental (i.e., cognitive) abilities. Statistics suggest that between 500,000 and 900,000 people are diagnosed with this disease every year, while several hundreds of thousands experience dementia or other aging-related cognitive decline.
While there are some available treatments designed to delay cognitive decline in individuals with mild or moderate AD symptoms, a cure for the disease has not yet been identified. A better understanding of the neural, genetic, cellular and molecular processes that contribute to the disease’s progression, as well as to neurodegeneration in general, could thus be highly valuable, as it could inform the future development of alternative treatments.
Past neuroscience research has identified the key role of microglia in AD. These are specialized immune cells that monitor the environment in the brain, clearing out damaged cells, debris and pathogens. The dysregulation of these cells has been linked to neurodegeneration and to the progression of AD.
Chronic kidney disease affects an estimated 37 million people in the U.S., and for many, there is no cure. But a new research project at Washington University in St. Louis seeks to change that by uncovering the mechanical basis of kidney cell injury.
To tackle chronic kidney disease, Guy Genin, the Harold and Kathleen Faught Professor of Mechanical Engineering at the WashU McKelvey School of Engineering, and Jeffrey Miner, the Eduardo and Judith Slatopolsky Professor of Medicine in Nephrology at WashU Medicine, teamed up with Hani Suleiman, an assistant professor of medicine at the University of Texas Southwestern Medical Center. The interdisciplinary team, with expertise spanning medicine, cell biology, genetics and engineering, received a five-year $4 million grant from the National Institute of Diabetes and Digestive and Kidney Diseases, part of the National Institutes of Health (NIH).
With the NIH’s support, the team plans to study the mechanobiology of podocytes, specialized cells in the kidney that help filter blood.
Researchers at Washington University in St. Louis have received a $4 million grant to study specialized cells that could help treat kidney disease.
World’s first pig lung transplant in brain-dead man lasts nine days in China.
In a medical first, a pig lung was transplanted into a brain-dead human, where it functioned for nine days.
Surgeons at Guangzhou Medical University, China, performed the cross-species lung transplantation.
The recipient, a 39-year-old man who had suffered a brain hemorrhage, received the left lung from a Chinese Bama Xiang pig that had undergone genetic modifications.
Translocations are chromosomal “cut and paste” errors that drive many lymphomas, a type of blood cancer and the sixth most common form of cancer overall. This includes mantle cell lymphoma, a rare but aggressive subtype diagnosed in about one in every 100,000 people each year.
A study by researchers at the Centre for Genomic Regulation (CRG) in Barcelona, has shown a new way translocations promote cancer. The translocation most typically found in mantle cell lymphoma drags a powerful regulatory element into a new area of the human genome, where its new position allows it to boost the activity of not just one but 50 genes at once.
The discovery of this genome rewiring mechanism shows the traditional focus on the handful of genes at chromosomal breakpoints is too narrow. The study also greatly expands the list of potential drug targets for mantle cell lymphoma, for which there is no known cure.
Scientists at the Medical Research Council’s Laboratory of Molecular Biology say they’ve engineered a bacteria whose genetic code is more efficient than any other lifeform on Earth.
They call their creation “Syn57,” a bioengineered strain of E. coli — yes, the same bad boy that can make you extremely sick if you eat an undercooked hot dog — which uses seven less codons than all life on earth. A codon, put simply, is a three-letter sequence found in DNA and RNA which delivers instructions for amino acids, a fundamental “building block” of life.
For the past billions years or so, all known life on earth has used 64 codons. Scientists cracked the code detailing which codons corresponded to which amino acids — mapping the standard genetic code, in other words — in 1966, revealing only 20 total amino acids.
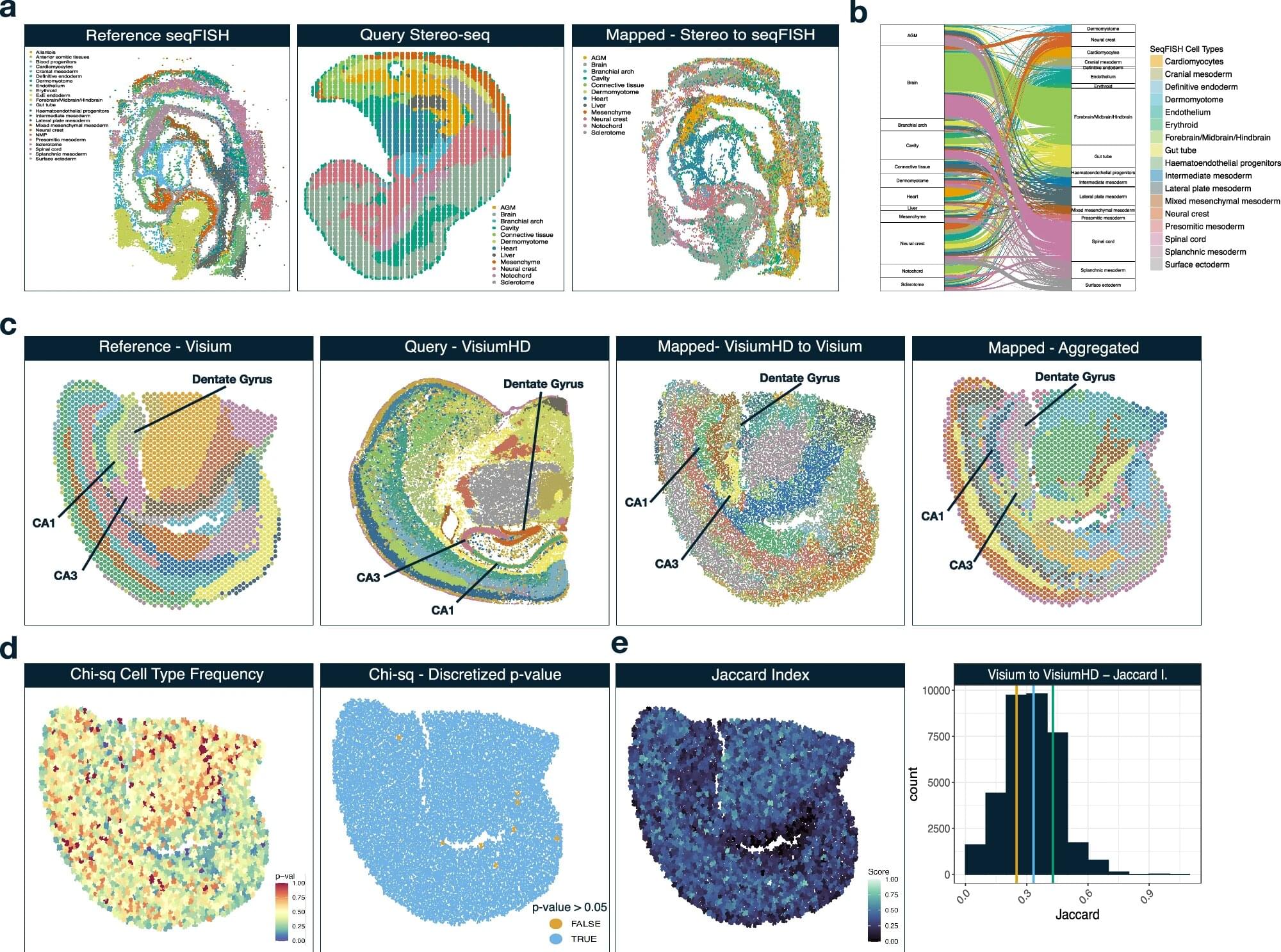
Researchers at VCU Massey Comprehensive Cancer Center have developed a new computational tool called Vesalius, which could help clinicians understand the complex relationships between cancer cells and their surrounding cells, leading to potential discoveries regarding the development of hard-to-treat cancers.
Findings from a new study, published in Nature Communications, could help guide the identification of predictive biomarkers for multiple cancers and better inform the effectiveness of different treatment options based on individuals’ specific type of disease.
Rajan Gogna, Ph.D., member of the Developmental Therapeutics research program at Massey and assistant professor in the VCU School of Medicine’s Department of Human and Molecular Genetics, and a team of collaborators were driven by the goal of interpreting extensive amounts of data in a meaningful way.