New research reveals that a gene mutation tied to Alzheimer’s disease disrupts the production of exosomes, tiny cellular particles essential for communication between brain cells.
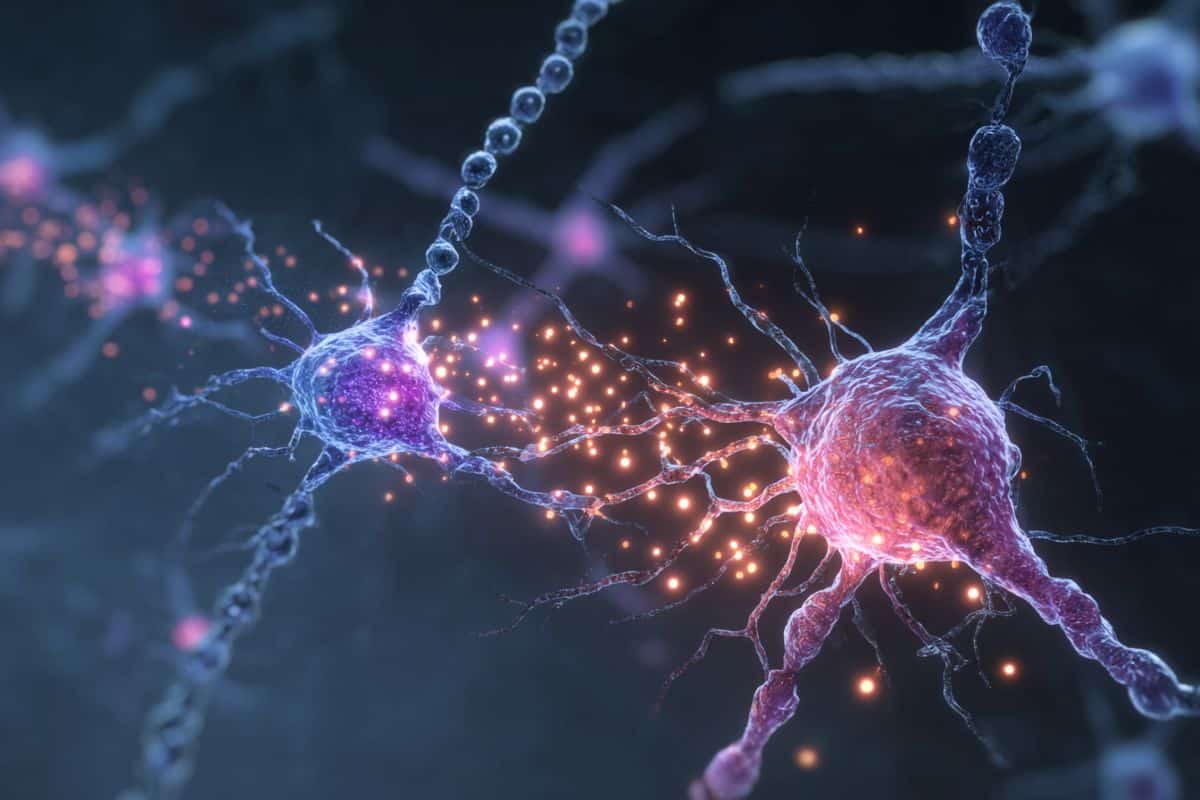

The cerebellum, a brain region located at the back of the head that has long been known to support the coordination of muscle movements, has recently also been implicated in more sophisticated mental functions. Purkinje cells are the only neurons located in the cerebellum that integrate information in the cerebellar cortex and send it to other parts of the nervous system.
Purkinje cells are large and highly branched nerve cells that can have different functions. While many past studies have explored the roles of these cells, the neural and genetic processes shaping their diversity have not yet been fully elucidated.
Researchers at the University of Connecticut School of Medicine recently carried out a study aimed at exploring the possible role of the FOXP genes, a family of genes known to contribute to switching other genes “on and off,” in shaping Purkinje cell populations and the formation of circuits in the cerebellum. Their findings, published in Nature Neuroscience, hint at the existence of at least 11 different Purkinje cell subtypes, suggesting that the FOXP1 and FOXP2 genes contribute to their diversification.
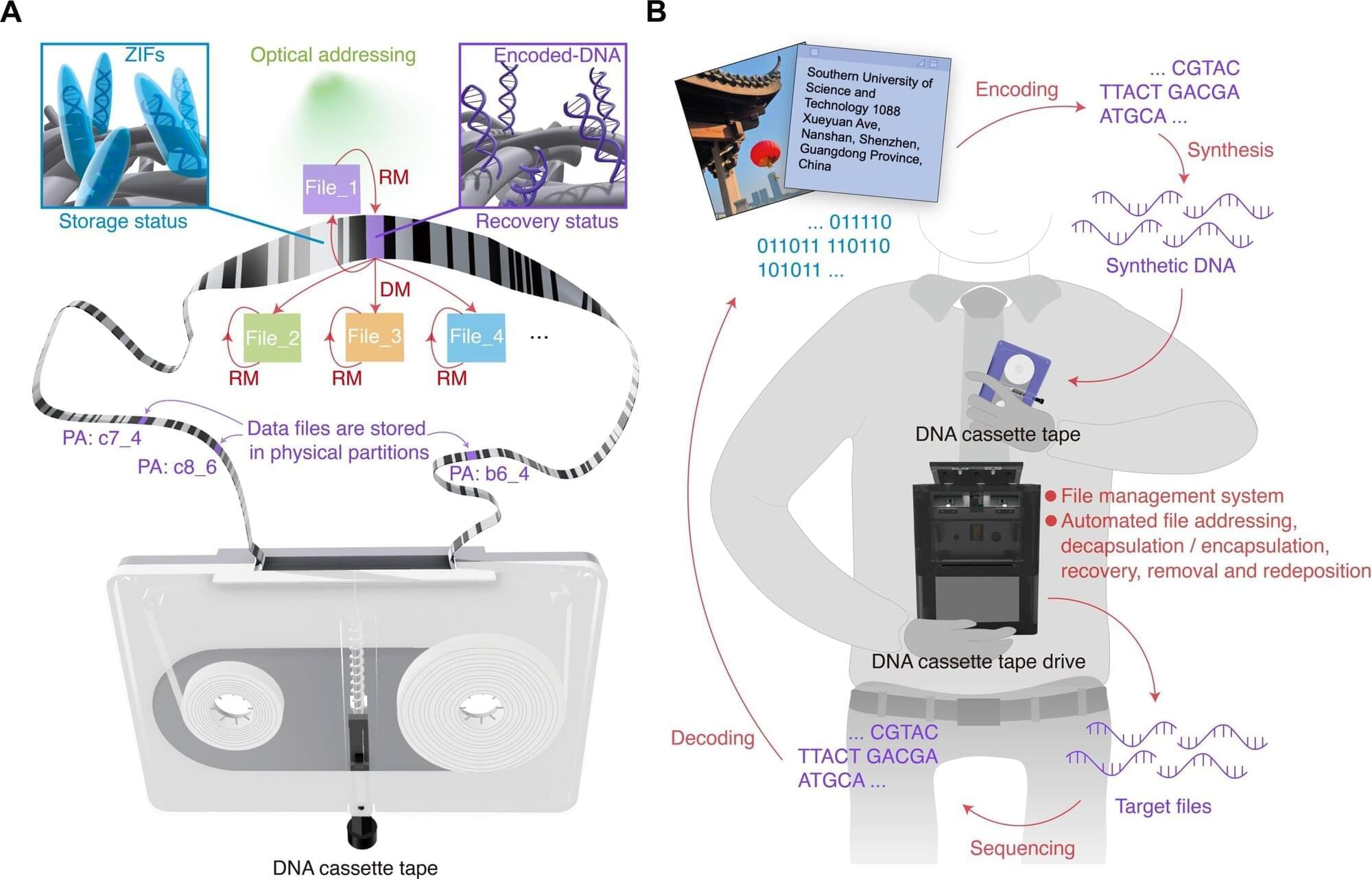
Our increasingly digitized world has a data storage problem. Hard drives and other storage media are reaching their limits, and we are creating data faster than we can store it. Fortunately, we don’t have to look too far for a solution, because nature already has a powerful storage medium with DNA (deoxyribonucleic acid). It is this genetic material that Xingyu Jiang at the Southern University of Science and Technology in China and colleagues are using to create DNA storage cassettes.

For the protein qubit to “encode” more information about what is going on inside a cell, the fluorescent protein needs to be genetically engineered to match the protein scientists want to observe in a given cell. The glowing protein is then attached to the target protein and zapped with a laser so it reaches a state of superposition, turning it into a nano-probe that picks up what is happening in the cell. From there, scientists can infer how a certain biological process happens, what the beginnings of a genetic disease look like, or how cells respond to certain treatments.
And eventually, this kind of sensing could be used in non-biological applications as well.
“Directed evolution on our EYFP qubit could be used to optimize its optical and spin properties and even reveal unexpected insights into qubit physics,” the researchers said. “Protein-based qubits are positioned to take advantage of techniques from both quantum information sciences and bioengineering, with potentially transformative possibilities in both fields.”

When cells are healthy, we don’t expect them to suddenly change cell types. A skin cell on your hand won’t naturally morph into a brain cell, and vice versa. That’s thanks to epigenetic memory, which enables the expression of various genes to “lock in” throughout a cell’s lifetime. Failure of this memory can lead to diseases, such as cancer.
Traditionally, scientists have thought that epigenetic memory locks genes either “on” or “off” — either fully activated or fully repressed, like a permanent Lite-Brite pattern. But MIT engineers have found that the picture has many more shades.
In a new study appearing today in Cell Genomics, the team reports that a cell’s memory is set not by on/off switching but through a more graded, dimmer-like dial of gene expression.
EVANSTON, IL. — With the power to rewrite the genetic code underlying countless diseases, CRISPR holds immense promise to revolutionize medicine. But until scientists can deliver its gene-editing machinery safely and efficiently into relevant cells and tissues, that promise will remain out of reach.
Now, Northwestern University chemists have unveiled a new type of nanostructure that dramatically improves CRISPR delivery and potentially extends its scope of utility.
Called lipid nanoparticle spherical nucleic acids (LNP-SNAs), these tiny structures carry the full set of CRISPR editing tools — Cas9 enzymes, guide RNA and a DNA repair template — wrapped in a dense, protective shell of DNA. Not only does this DNA coating shield its cargo, but it also dictates which organs and tissues the LNP-SNAs travel to and makes it easier for them to enter cells.
New system delivers CRISPR machinery more safely and effectively into cells.
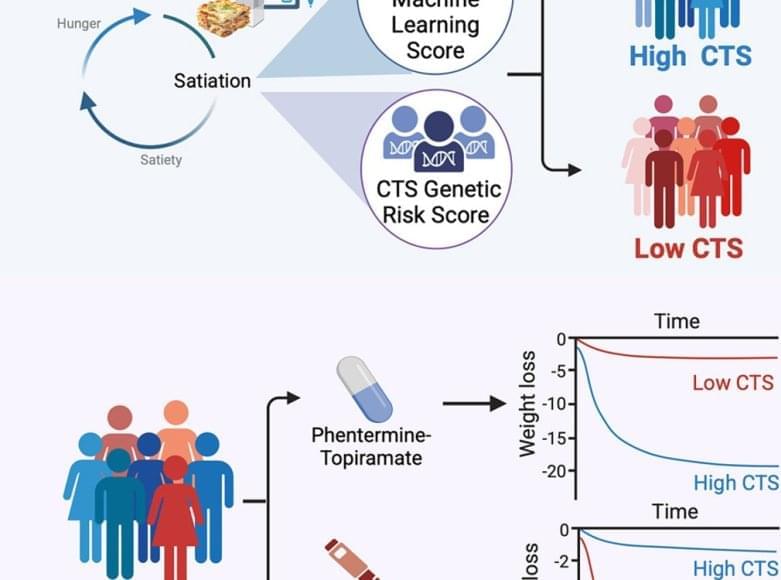
Meal size and termination is regulated by a process called satiation, which varies widely among adults with obesity.
The researchers assessed calories to satiation (CTS) and integrated a machine learning genetic risk score (CTSGRS) to predict obesity treatment outcomes.
High CTS or CTSGRS identified individuals who responded better to phentermine-topiramate, whereas low CTS or CTSGRS predicted greater weight loss with liraglutide, highlighting personalized obesity therapy.